Author: Alisha G C
Abstract
Blinatumomab is a first-in-class bispecific T-cell engager (BiTE) antibody that has fundamentally reshaped the therapeutic landscape of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). By physically linking endogenous cytotoxic T cells to CD19-expressing malignant B cells, Blinatumomab enables potent, major histocompatibility complex (MHC)–independent T-cell cytotoxicity, thereby overcoming critical limitations of conventional chemotherapy and antigen presentation–dependent immunotherapies. Despite impressive clinical efficacy, particularly in minimal residual disease (MRD)–positive and heavily pretreated patients, therapeutic resistance and disease relapse remain significant barriers to durable remission. This comprehensive review provides an in-depth analysis of Blinatumomab’s molecular architecture, immunologic mechanism of action, pharmacokinetics, clinical outcomes, resistance pathways, and emerging strategies to overcome therapeutic failure, integrating insights from structural biology, immunology, and translational research.
Molecular Structure of Blinatumomab
Blinatumomab is a recombinant fusion protein composed of two single-chain variable fragments (scFvs) derived from monoclonal antibodies targeting CD19 and CD3ε, respectively. Unlike conventional monoclonal antibodies, BiTE molecules are compact, flexible, and engineered to bring immune effector cells into direct contact with tumor cells.
CD19 scFv: The B-Cell Targeting Domain
CD19 is a 95 kDa type I transmembrane glycoprotein belonging to the immunoglobulin superfamily and is expressed throughout most stages of B-cell development, from pre-B cells to mature B lymphocytes. Functionally, CD19 acts as a coreceptor of the B-cell receptor (BCR) complex, where it amplifies signaling by lowering activation thresholds and regulating downstream pathways such as PI3K–AKT and SYK signaling.
From a therapeutic perspective, CD19 is an ideal immunotherapy target due to:
Expression in >95% of B-ALL cases
Stable surface localization during leukemogenesis
Minimal expression outside the B-cell lineage, reducing off-target toxicity
The CD19 scFv in Blinatumomab recognizes an extracellular epitope that remains accessible even in low-antigen–density states, enabling efficient engagement of malignant B cells.
CD3ε scFv: The T-Cell Engagement Domain
CD3ε is an essential signaling component of the T-cell receptor (TCR) complex, associated with CD3γ, CD3δ, and the CD3ζ homodimer. Upon engagement, CD3ε transduces activation signals through immunoreceptor tyrosine-based activation motifs (ITAMs) present on CD3ζ chains.
Importantly, Blinatumomab binds CD3ε independently of antigen specificity, allowing recruitment of polyclonal, non–tumor-specific T cells. This mechanism bypasses the need for peptide antigen processing and presentation, a frequent immune-evasion strategy in leukemia.
Linker Design and Structural Configuration
The two scFvs are connected via a flexible, non-immunogenic (Gly₄Ser)₃ linker, producing a ~55 kDa BiTE molecule. This linker provides sufficient rotational freedom to allow simultaneous binding of CD19 and CD3 without steric hindrance.
A defining feature of Blinatumomab is the absence of an Fc domain, which:
Enhances tissue penetration and diffusion
Eliminates Fcγ receptor binding
Reduces off-target immune activation, including ADCC and CDC
Minimizes nonspecific cytokine release
Key Structural Features
Monovalent scFv binding enables rapid serial engagement of multiple tumor cells
Flexible linker architecture supports stable immunologic synapse formation
Short serum half-life (~2 hours) allows precise pharmacokinetic control via continuous IV infusion
Fc-less design improves safety and specificity
Crystallographic and biophysical studies confirm that the spatial orientation of the CD19 and CD3 binding arms is optimal for intercellular tethering, resulting in efficient formation of a functional cytolytic immunologic synapse.
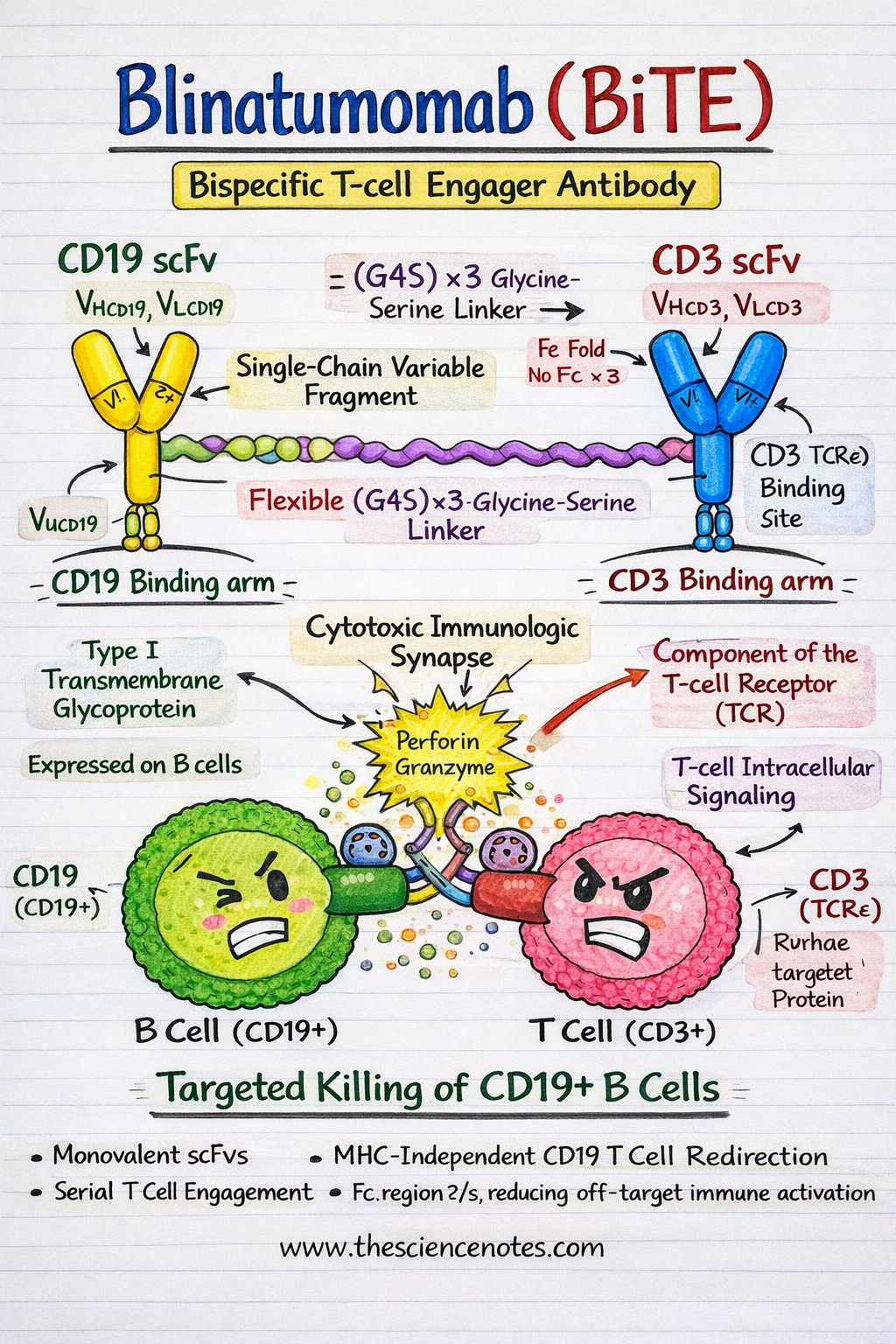
Mechanism of Action of Blinatumomab
1. Immunologic Synapse Formation and T-Cell Redirection
Blinatumomab mediates MHC-independent T-cell cytotoxicity by physically bridging CD3+ T cells and CD19+ leukemic B cells, effectively converting resting T cells into serial tumor killers.
Bipartite engagement induces TCR clustering through CD3ε while anchoring malignant B cells via CD19
This forced proximity mimics physiological immune synapse formation
At the synapse, key signaling molecules such as Lck, LAT, SLP-76, PKCθ, and actin-regulatory proteins are recruited and spatially organized.
Intracellular Signal Transduction
Upon CD3 engagement:
Lck phosphorylates ITAMs on CD3ζ chains
ZAP-70 is recruited and activated
Adaptor proteins (LAT, SLP-76) assemble signaling complexes
Calcium influx and diacylglycerol (DAG) production activate:
Calcineurin → NFAT
PKCθ → NF-κB
MAPK cascade → AP-1
These transcriptional programs drive T-cell activation, proliferation, and cytotoxic function.
Effector Functions
Perforin–granzyme B–mediated apoptosis
Release of pro-inflammatory cytokines (IFN-γ, IL-2, TNF-α)
Serial killing, where a single T cell disengages and targets multiple leukemic cells
2. T-Cell Reprogramming and Immune Memory
Beyond immediate cytotoxicity, Blinatumomab induces functional reprogramming of T cells, promoting expansion of:
Effector memory T cells (T_EM)
Central memory T cells (T_CM)
These populations contribute to long-term immune surveillance and may underlie sustained MRD negativity observed in responding patients.
3. Pharmacokinetics and Immune Dynamics
Due to its small size and lack of Fc region, Blinatumomab exhibits:
Rapid clearance via renal filtration and proteolysis
Peak T-cell activation and expansion between days 7–14
Preferential expansion of CD8+ cytotoxic T cells, correlating with depth of response
Continuous intravenous infusion ensures stable plasma concentrations while allowing rapid cessation in the event of toxicity.
Clinical Efficacy of Blinatumomab in B-ALL
TOWER Trial (NEJM, 2017)
Adult relapsed/refractory B-ALL
CR/CRh: 43% vs 25% (Blinatumomab vs chemotherapy)
Median overall survival: 7.7 vs 4.0 months
BLAST Trial (Blood, 2018)
MRD-positive B-ALL in hematologic remission
MRD clearance: 78% after one cycle
3-year overall survival: 71%
Adverse Events
Cytokine release syndrome (CRS): early onset, typically steroid-responsive
Neurotoxicity: grade ≥3 in ~10%, potentially linked to T-cell trafficking across the blood–brain barrier
Mechanisms of Resistance to Blinatumomab
1. Antigen Escape (CD19 Loss)
Alternative splicing (e.g., exon 2 skipping)
CD19 gene mutations or deletions
Lineage switch to myeloid phenotype, particularly in MLL-rearranged leukemia
2. T-Cell Intrinsic Resistance
Upregulation of exhaustion markers (PD-1, TIM-3, LAG-3)
TOX-dependent epigenetic reprogramming
Metabolic insufficiency and mitochondrial dysfunction
3. Immune Checkpoint and Microenvironmental Suppression
PD-L1 upregulation on leukemic blasts and MDSCs
Immunosuppressive cytokines (IL-10, TGF-β)
Expansion of regulatory T cells and suppressive monocytes
Strategies to Overcome Blinatumomab Resistance
Dual and Multispecific Targeting
CD19/CD22 BiTEs and CAR-T therapies
Reduced antigen-negative relapse
Checkpoint Inhibition
Combination with anti–PD-1/PD-L1 antibodies
Enhanced T-cell persistence and cytotoxicity
T-Cell Fitness Enhancement
IL-7 and IL-15 cytokine agonists
Allogeneic T-cell supplementation
Next-Generation BiTE Platforms
Half-life–extended BiTEs
Trispecific T-cell engagers incorporating costimulatory signals such as 4-1BB
Conclusion
Blinatumomab represents a paradigm shift in B-ALL immunotherapy, enabling precise, MHC-independent T-cell redirection with substantial clinical benefit across multiple disease settings. While resistance mechanisms such as antigen escape, T-cell dysfunction, and immune suppression remain significant obstacles, rational combination therapies, next-generation BiTE engineering, and immune profiling–guided strategies offer promising avenues to enhance durability of response. Continued integration of molecular biology, immunology, and clinical innovation will be critical to expanding the therapeutic window of Blinatumomab and achieving long-term cures in B-ALL.
Frequently Asked Questions (FAQ)
Q1. What is Blinatumomab and how does it work?
Blinatumomab is a bispecific T-cell engager (BiTE) antibody that links CD3-positive T cells to CD19-positive B cells, triggering MHC-independent T-cell–mediated cytotoxicity.
Q2. Why is CD19 an ideal target in B-ALL?
CD19 is expressed in over 95% of B-cell acute lymphoblastic leukemia cases and is absent from most non–B-lineage tissues, minimizing off-target toxicity.
Q3. What are the main resistance mechanisms to Blinatumomab?
Resistance arises from CD19 antigen loss, T-cell exhaustion, immune checkpoint upregulation, and suppressive tumor microenvironment factors.
Q4. How does Blinatumomab differ from CAR-T cell therapy?
Unlike CAR-T therapy, Blinatumomab redirects endogenous T cells without genetic modification and allows precise pharmacokinetic control through continuous infusion.
Q5. What are emerging strategies to overcome Blinatumomab resistance?
Approaches include dual-target BiTEs, checkpoint inhibitor combinations, cytokine-based T-cell support, and next-generation trispecific engager platforms.