Glassware is one of the most recognizable and essential components of a professional chemistry laboratory. From simple beakers to highly precise volumetric flasks, laboratory glassware enables scientists to measure, mix, heat, and observe chemical reactions safely and accurately. Despite the growing use of plastics and alternative materials, glass remains the gold standard in laboratory work due to its durability, chemical resistance, and precision.
This study guide explains laboratory glassware materials, principles of accuracy, connections and sealing, cleaning methods, and common types of glassware used in chemistry labs.
Why Is Glassware Important in the Chemistry Laboratory?
Glassware is widely used in chemistry because it offers several advantages:
Relatively low cost
Excellent chemical resistance
Ability to withstand high temperatures
Transparency for easy observation
Availability in high-precision volumetric forms
Although plastics and even everyday kitchen materials are sometimes used for simple tasks, glass sets the standard for laboratory techniques and experimental accuracy.
Types of Glass Used in Laboratory Glassware
Not all laboratory glass is the same. Different applications require different glass compositions.
1. Soda-Lime (Float) Glass
Soda-lime glass is the most common consumer-grade glass. While it is suitable for many everyday applications, it cracks under rapid heating or cooling due to thermal expansion and contraction. For this reason, it is rarely used in professional chemistry labs.
2. Borosilicate Glass
To overcome thermal stress issues, laboratories primarily use borosilicate glass, which contains small amounts of boron. This gives it a low coefficient of thermal expansion, preventing cracking during heating and cooling cycles.
Most common trade name: Pyrex
Used in beakers, flasks, and graduated cylinders
Thermally robust but still contains minor impurities
A simple way to identify borosilicate glass is by looking down its long axis—a faint greenish tint indicates impurities.
3. Fused Silica (Quartz Glass)
When extreme conditions are required, fused silica (fused quartz) is used.
Made of chemically pure silicon dioxide
Melting point above 1,600 °C
Transparent to UV light
Used above 450 °C
Fused silica is optically clear and completely colorless, making it easy to distinguish from borosilicate glass.
Principles of Accuracy in Laboratory Glassware
Not all glassware is designed for precise measurements.
Standard Glassware Accuracy
Beakers and flasks typically measure volume with ±5% accuracy. These are suitable for qualitative work where exact volumes are not critical.
Volumetric Glassware
Volumetric glassware is designed for high precision, often to four significant figures. Accuracy is indicated by:
Etched calibration line
Calibration temperature (usually 20 °C)
TD or TC marking
TD (To Deliver): accurately dispenses the stated volume
TC (To Contain): contains the stated volume but may not fully transfer
Understanding these markings is essential for proper laboratory technique.
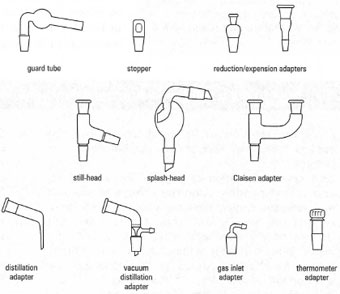
Sealing and Connecting Glassware
Glassware is often sealed or connected using specialized components.
Stoppers and Septa
Rubber, cork, neoprene, and Teflon stoppers
Conical shape creates a tight wedge seal
Available with 0–3 holes for tubing or thermometers
Septum stoppers allow syringe access
Rubber stoppers are sized from 000–10, while glass stoppers use standardized dimensions (e.g., 24/40).
Ground Glass Joints
Common joint types include:
Standard taper
Ball-and-socket
O-ring joints
While these joints seal well, they can seize. Joint grease (vacuum grease or Krytox) prevents sticking. Since joints are not mechanically strong, Keck clips or springs are used to secure connections.
Clamping and Supporting Glassware
Proper support is critical for safe experiments.
Ring stands and metal clamps support flasks
Three-finger clamps attach to glass necks
Round-bottom flasks always require clamps
Lab jacks allow vertical adjustment of heavy setups
Cork rings stabilize round-bottom flasks on benches
Even flat-bottom glassware can tip easily, especially during vacuum filtration.
Cleaning Laboratory Glassware
Cleaning ensures accuracy and safety.
Routine Cleaning
Soap and water are usually sufficient
Acetone removes sticky organic residues
Chemical Cleaning Baths
Base bath (ethanol + sodium hydroxide): removes organic carbon deposits
⚠️ Never use on volumetric glasswareAcid bath (dilute hydrochloric acid): removes metal contamination
Effective cleaning often requires 24–48 hours of soaking.
Common Types of Laboratory Glassware
1. Qualitative Glassware
Beakers: holding, mixing, pouring liquids
Erlenmeyer flasks: swirling, heating, reflux
Florence flasks: boiling and heating
Test tubes: small-scale reactions
Watch glasses: evaporation and crystallization
Crystallization dishes: high surface-area evaporation
2. Measuring Glassware
Graduated cylinders: semi-precise measurements (~1%)
Volumetric flasks: preparing standardized solutions
Volumetric pipettes: precise liquid transfer
Micropipettes: 1 µL–1,000 µL volumes
Burettes: titration experiments
3. Procedural Glassware and Ceramics
Round-bottom flasks: synthesis and heating
Separatory funnels: liquid–liquid separation
Büchner flasks and funnels: vacuum filtration
Crucibles: high-temperature heating
Mortar and pestle: grinding solids
Applications and Summary
Each piece of laboratory glassware is designed for a specific purpose, but flexibility exists depending on experimental needs. With proper technique, sealing, support, and cleaning, glassware enables safe and accurate laboratory work. Specialized setups can even be custom-made by professional glassblowers.
Conclusion
Laboratory glassware is fundamental to chemistry education and research. Understanding glass types, precision markings, connections, and applications helps students develop strong laboratory skills and good experimental habits. Despite modern alternatives, glassware remains the backbone of laboratory science.