All matter in the universe is composed of atoms, the smallest individual units of elements. Although atoms were once thought to be indivisible, scientists now know that they are made up of even smaller components called subatomic particles. The structure and behavior of atoms explain the physical and chemical properties of all substances, from simple gases to complex living organisms.
Modern chemistry and physics are built upon the understanding of atomic structure. By studying atoms, scientists can explain how elements interact, how compounds form, and why matter behaves the way it does.
What Is an Atom?
An atom is the smallest unit of an element that retains that element’s chemical identity. Each atom consists of three primary subatomic particles:
Protons
Neutrons
Electrons
Together, these particles account for the mass and electrical charge of an atom. While atoms cannot be broken down by ordinary chemical reactions, their internal structure determines how they interact with other atoms.
Each element—such as hydrogen, oxygen, or potassium—consists of atoms with a unique internal structure, especially in the number of protons found in the nucleus.
The History of Atomic Theory
Early Ideas: Democritus and Atomos
The concept of atoms dates back to around 450 B.C., when the Greek philosopher Democritus proposed that all matter was made of tiny, indivisible particles. He called these particles atomos, meaning “indivisible.” Although groundbreaking, his idea lacked experimental evidence and was largely ignored for centuries.
Dalton’s Atomic Theory
Atomic theory was revived in the early 19th century by English scientist John Dalton, whose ideas form the foundation of modern atomic theory. Dalton proposed five key postulates:
All matter is composed of tiny particles called atoms.
All atoms of a given element are identical.
Atoms of different elements differ from one another.
Atoms of different elements combine in fixed ratios to form compounds.
Atoms cannot be created or destroyed in chemical reactions, only rearranged.
While later discoveries refined some of these ideas, Dalton’s theory was essential in establishing atoms as the basis of matter.
Discovering Subatomic Particles
Dalton believed atoms were indivisible, but scientific discoveries in the late 19th and early 20th centuries proved otherwise.
The Discovery of the Electron
In 1897, J.J. Thomson discovered the electron, a negatively charged particle much smaller than an atom. Since atoms are electrically neutral overall, this discovery raised an important question: how could negative particles exist inside a neutral atom?
Thomson proposed the plum pudding model, which suggested that electrons were embedded within a positively charged “pudding.” Although incorrect, this model marked the first attempt to describe atomic structure.
Rutherford’s Nuclear Model
Just a few years later, Ernest Rutherford conducted experiments that dramatically changed the understanding of atomic structure. His gold foil experiment showed that:
Most of an atom’s mass is concentrated in a tiny central nucleus
The nucleus carries a positive charge
Electrons occupy the space surrounding the nucleus
This discovery disproved the plum pudding model and revealed that atoms are mostly empty space.
The Discovery of the Neutron
In 1932, James Chadwick discovered the neutron, a neutral particle found in the nucleus. This discovery completed the basic picture of atomic structure, explaining how atoms could have additional mass without additional charge.
The Structure of an Atom
Atoms consist of a central nucleus surrounded by electrons moving in a cloud-like region. Each subatomic particle has distinct properties and functions.
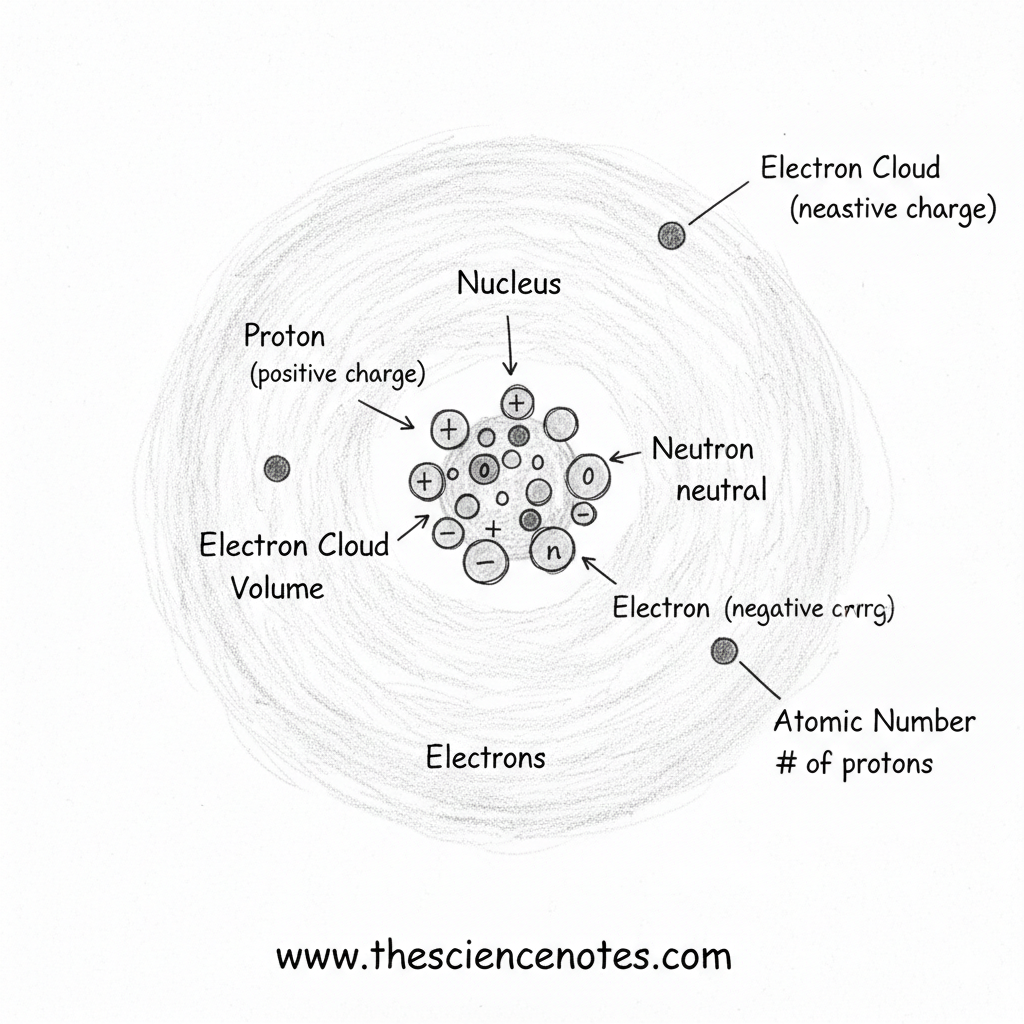
Protons: Defining the Element
Protons are positively charged particles located in the nucleus of an atom. Each proton has a mass of one atomic mass unit (AMU).
The number of protons in an atom is known as its atomic number, which determines the identity of the element. For example:
Hydrogen has 1 proton
Carbon has 6 protons
Oxygen has 8 protons
Changing the number of protons changes the element itself.
Neutrons: Adding Mass and Stability
Neutrons are also found in the nucleus and have no electrical charge. Like protons, they have a mass of one AMU, contributing significantly to the atom’s total mass.
Neutrons play an important role in stabilizing the nucleus. Atoms of the same element can have different numbers of neutrons, forming isotopes with slightly different atomic masses.
Electrons: The Cloud Around the Nucleus
Electrons are negatively charged particles that move rapidly around the nucleus in regions known as electron clouds. These clouds account for most of an atom’s volume, even though electrons have negligible mass.
Electrons remain near the nucleus due to their attraction to the positively charged protons. Their arrangement, known as electron configuration, determines how atoms interact and bond with one another.
Atomic Mass and Charge
The atomic mass of an atom is primarily determined by the combined mass of its protons and neutrons. Electrons contribute very little to mass but play a crucial role in charge balance.
In a neutral atom:
Number of protons = number of electrons
Positive and negative charges cancel out
This balance gives the atom no net electrical charge.
Ions and Electron Configuration
Atoms are not always electrically neutral. If an atom gains or loses electrons, it becomes an ion.
Cations are positively charged ions formed when atoms lose electrons.
Anions are negatively charged ions formed when atoms gain electrons.
Ion formation is critical in chemical reactions, biological processes, and electrical conductivity.
Why Atomic Structure Matters
Understanding atomic structure helps explain:
Chemical reactions and bonding
Physical properties of substances
Electrical behavior of materials
Biological processes at the molecular level
From the formation of water molecules to the transmission of nerve impulses, atomic interactions shape the natural world.
Conclusion
Atoms are the fundamental units of matter, composed of protons, neutrons, and electrons. Centuries of scientific discovery—from Democritus to Dalton to modern physicists—have revealed the intricate structure hidden within these tiny particles.
By understanding atomic structure, scientists can explain the behavior of elements, predict chemical reactions, and unlock new technologies. Though atoms are incredibly small, their impact on the universe is immense.