Chemistry is the scientific study of matter, including its structure, properties, and the changes it undergoes. At the foundation of chemistry lies the concept of atoms and elements. These fundamental units play a crucial role in the chemistry of life, particularly in understanding the composition and function of the human body. This note explores the basic structure of atoms, the distinction between atoms and elements, the major elements in the human body, and the role of isotopes and radioisotopes in chemistry.
Atoms and Atomic Structure
Atoms are the smallest units of matter that retain the chemical properties of an element. They consist of three primary subatomic particles: protons, neutrons, and electrons.
- Protons are positively charged particles located in the atomic nucleus. They have a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom defines the element and is known as the atomic number. For example, all carbon atoms have 6 protons.
- Neutrons are neutral particles (i.e., they have no charge) that also reside in the nucleus. They are slightly larger than protons and have a mass of about 1 amu. Neutrons contribute to the atomic mass but do not affect the atom’s charge.
- Electrons are negatively charged particles with negligible mass compared to protons and neutrons. They orbit the nucleus in regions called electron shells. Despite their minimal mass, electrons are crucial in chemical bonding and reactions.
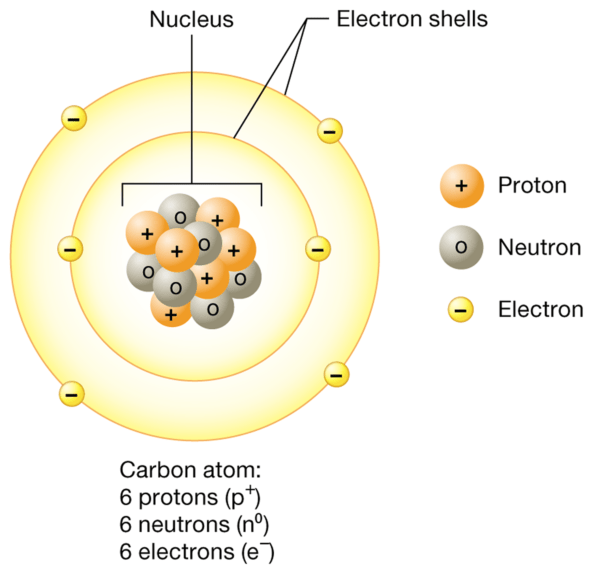
The nucleus, which contains protons and neutrons, is incredibly dense compared to the electron shells, which are relatively vast. Although electrons are spread over a large volume, most of an atom’s mass is concentrated in the nucleus.
Electron Shells
The classical model of the atom depicts electrons orbiting the nucleus in a manner similar to planets around the sun. However, this is a simplification. Electrons exist in regions of probability known as electron shells. The first shell can hold up to 2 electrons, the second shell up to 8, and the third shell up to 18. Electrons fill the lowest energy levels (or shells) first before occupying higher ones. This arrangement influences how atoms interact chemically.
Atoms vs. Elements
The terms “atom” and “element” are often used interchangeably but have distinct meanings:
- Atom: An atom is the smallest unit of an element that retains its chemical properties. It consists of protons, neutrons, and electrons. Atoms can combine to form molecules, which are the building blocks of matter.
- Element: An element is a pure substance consisting of only one type of atom. Elements are defined by their atomic number, which is the number of protons in their atoms. For instance, hydrogen (H) is an element with atoms that have one proton. There are currently 118 known elements, each with unique properties.
Major Elements in the Human Body
The human body is primarily composed of a few key elements, which are essential for various biological functions:
- Oxygen (O): Making up about 65% of the human body by mass, oxygen is crucial for cellular respiration, the process by which cells generate energy.
- Carbon (C): Comprising approximately 18% of the body, carbon is the backbone of organic molecules, including carbohydrates, proteins, lipids, and nucleic acids.
- Hydrogen (H): Accounting for about 10% of the body, hydrogen is a component of water and organic molecules, playing a vital role in maintaining pH balance and energy production.
- Nitrogen (N): Making up about 3% of the body, nitrogen is a key element in amino acids, which are the building blocks of proteins, and in nucleic acids, which make up DNA and RNA.
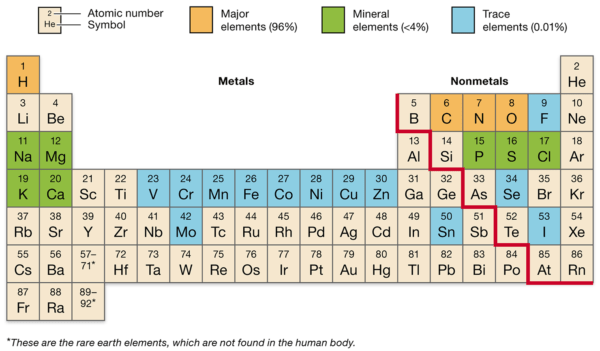
Other elements, such as calcium, phosphorus, potassium, sulfur, sodium, and magnesium, are present in smaller quantities but are still essential for maintaining health and supporting various physiological functions.
Atomic Number, Mass Number, Isotopes, and Radioisotopes
To understand the variation among atoms, we need to distinguish between atomic number, mass number, isotopes, and radioisotopes:
- Atomic Number: The atomic number is the number of protons in the nucleus of an atom. It defines the element and its position on the periodic table. For instance, the atomic number of carbon is 6, meaning all carbon atoms have six protons.
- Mass Number: The mass number is the sum of protons and neutrons in an atom’s nucleus. It represents the atom’s total nuclear mass. For example, carbon-12 has a mass number of 12, indicating a total of 6 protons and 6 neutrons.
- Isotopes: Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. This results in different mass numbers. For example, carbon has three isotopes: carbon-12 (6 protons and 6 neutrons), carbon-13 (6 protons and 7 neutrons), and carbon-14 (6 protons and 8 neutrons).
- Radioisotopes: Radioisotopes, or radioactive isotopes, are unstable isotopes that decay over time, emitting radiation in the process. This radioactive decay changes the isotope into a more stable form. Radioisotopes are used in various applications, including medical diagnostics and treatment. For example, iodine-131 is used in treating thyroid disorders.
Production of Isotopes
Isotopes are produced through various nuclear processes:
- Natural Processes: Some isotopes occur naturally. For instance, carbon-14 is continuously formed in the atmosphere through the interaction of cosmic rays with nitrogen-14.
- Artificial Processes: Isotopes can also be artificially created in laboratories or nuclear reactors. By bombarding stable atoms with neutrons or other particles, scientists can produce isotopes with different mass numbers. For instance, technetium-99m, used in medical imaging, is produced in nuclear reactors.
Conclusion
Understanding the basic concepts of atomic structure, the distinction between atoms and elements, and the roles of isotopes and radioisotopes is fundamental in chemistry and essential for exploring the chemistry of life. These concepts help elucidate the composition and function of biological molecules and the use of radioactive materials in medicine. By grasping these principles, we gain insights into how the smallest units of matter contribute to the complex processes that sustain life.