Crude protein content determination is an essential aspect spanning multiple domains like agriculture, nutrition, and food science, serving a pivotal role in evaluating the nutritional composition of substances, particularly in the context of food and feed. The process of assessing crude protein content involves employing laboratory analytical techniques, with the Kjeldahl method emerging as one of the most prominent approaches. This method entails the digestion of a sample and the subsequent quantification of its nitrogen content, where nitrogen acts as a surrogate for measuring protein concentration. By applying a conversion factor, often 6.25, to the nitrogen concentration, we arrive at an estimation of the crude protein content. However, it’s noteworthy that the term ‘crude’ conveys an approximation, lacking specific insights into the particular protein types present or their nutritional qualities. Within this comprehensive guide, we will delve into the fundamental principles, methodologies, and diverse applications of crude protein analysis through the Kjeldahl method, shedding light on its significance across various industries and fields.
What is crude protein?
- Crude protein content is typically expressed as a percentage of the total weight of the substance being analyzed.
- It is determined through a laboratory analysis method called the Kjeldahl method or the Dumas method, which involves digesting the sample and then quantifying the nitrogen content.
- Since nitrogen is a component of protein molecules, the nitrogen concentration is utilized as a stand-in for protein concentration estimation.
- To calculate the crude protein content, the nitrogen concentration is multiplied by a conversion factor (often 6.25).
- The term “crude protein content” refers to a measurement that is used in various kinds of fields of study, such as agriculture, nutrition, and food science, to estimate the approximate protein content of a substance, usually a component in food or feed.
- The term “crude” indicates that the measurement is only an approximate estimation of the protein content and does not give specific information about the various types of proteins that are present or their nutritional value.
Learn more about Food Analysis
- Determination of Crude Fat Analysis in Food samples (thesciencenotes.com)
- Determination of Crude Fiber in Food Sample – The Science Notes
- Determination of iodine value of fats and oils – The Science Notes
- Determination of saponification value of oil or fats sample – The Science Notes
Principle of crude protein content determination
The Kjeldahl procedure can be basically divided into three parts: (1) digestion, (2) distillation, (3) titration. At a temperature of about 370 °C, organic nitrogen is transformed into ammonium during the digestion process. Using a copper-based catalyst, the sample is digested in H2SO4 for this experiment, converting N into NH3 that is then distilled and titrated.
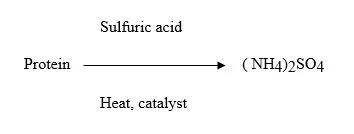
The digested sample is made alkaline with NaOH during the distillation process, and the nitrogen is removed as NH3. This NH3 is trapped in a boric acid solution.
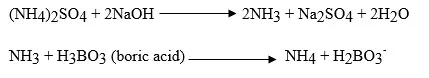
The amount of ammonia nitrogen in this solution is quantified by titration with a standard HCl solution. The amount of HCl titrant needed for the reagent blank that is carried during the analysis is eliminated from each determination.

This analysis determines total nitrogen and not usable nitrogen, and this is the reason it is called a crude protein analysis.
Apparatus required for Crude protein content determination.
- Balance machine
- Acid proof gloves
- Volumetric flask
- Funnel
- Pipette filler
- Conical flask
- Measuring cylinder
- Kendahl’s flask
- Distillation unit
- Digestion unit
Procedure of Crude protein content determination by the Kjeldahl methods
Step 1: Digestion
- Gather everything needed for the sample preparation.
- Before doing the weighting, the balance’s calibration status should be checked.
- Place a weighting boat on the balance machine and close the door and tare the weight.
- Use a clean spatula to transfer the sample into boat.
- Homogenized portion of sample should be transfer quickly.
- Take about 2g of the sample to the weighted boat.
- Note the sample weight.
- Take the weighted sample into the kjeldhal flask.
- Again, weight 2gm of catalyst.
- Add the catalyst to the same flask with the sample to be mixed.
- Now, using a pipette, measure 20 ml of concentrated sulfuric acid.
- Pour the acid into the sample flask.
- Shake the flask gently to mix the acid with sample and catalyst.
- Finally, place the flask on the digestion unit carefully.
- Set the temperature of the digester to 230 degrees Celsius and turn on the power supply.
- water circulation pipe should be turned on.
- For two hours, run the digester.
- After two hours, a clear green solution shows that digestion is complete.
- Now turn off the digester and let the flask cool.
- Now, the digested sample will be diluted with water.
- Add approximately 20ml of distilled water into the flask.
- The digested sample should be mixed and poured into a 100ml volumetric flask.
- To make sure that no digested material is left to transfer, repeat the process three times.
- Now, add distilled water to make final volume of 100ml.
Step 2: Distillation
- Measure 30ml of 4% boric acid.
- The boric acid solution is to be poured into a conical flask.
- Set the flask on the distillate collection apparatus.
- Set up all other apparatus with the distillation unit.
- Mix the digested sample by rotating.
- Transfer 10ml of the digested sample into a distillation flask.
- Add 50ml of 40% sodium hydroxide at this stage.
- Also add another 50ml of distilled water.
- Close the valve after opening it to allow the mixture to drain into the flask’s bottom.
- Distillation should be carried out at 200 degrees Celsius.
- After collecting approximately 100 cc of distillate after 1 hour, stop the distillation.
Step 3: Titration
- Take 0.1N HCL into burette.
- Note the burette’s initial reading.
- Add few drops of methyl red indicator.
- Mix well and start titration adding 0.1N HCL.
- If the color turns orange, stop the titration.
- Note the burette’s final reading.
Step 4: Calculation
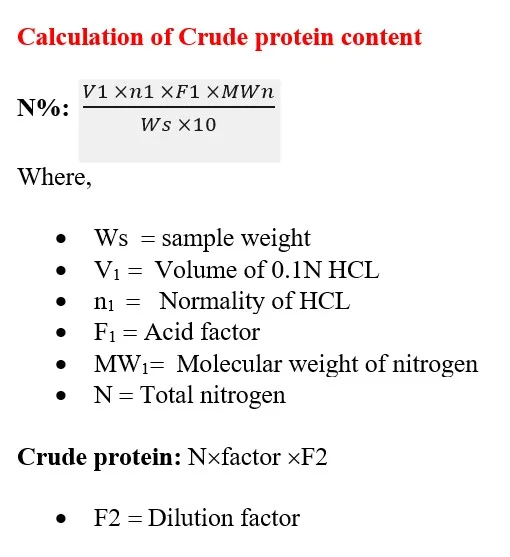
Application of Crude protein content determination
The determination of crude protein content has various applications across different industries and fields, reflecting its importance in assessing nutritional value, quality, and compliance with regulations. The following are some significant uses for calculating crude protein content:
Food industry
- Nutritional Labeling: For correct nutritional labeling, food producers use crude protein content analysis to determine the protein composition of their products.
- Product Quality: Food goods like meat, dairy, cereals, and processed foods benefit from consistency and quality control.
- Product Development: To develop new food products with precise nutritional profiles, researchers and product developers use information on the protein composition of existing foods.
Animals’ nutrition’s
- Diet Formulation: Formulating a balanced diet for livestock, poultry, and fishing requires analyzing the crude protein level to make sure the animals are getting enough protein for development and health.
- Feed Quality Control: It is used by feed producers to ensure that animal feeds are of high quality and follow to specified nutritional standards.
Agriculture
- Crop selection: Farmers choose varieties of crops with higher protein contents for certain applications, such as wheat for bread or barley for animal feed, using data on protein content.
- Harvest Timing: The best nutritional quality for livestock feed is ensured by harvesting forage crops at an appropriate time based on their protein content.
Research and developments
- Nutritional studies: To determine the effects of various diets on human and animal health, researchers analyze the crude protein content of food.
- Plant breeding: It supports efforts to generate crops with increased protein content or better protein quality.
Medical and clinical application
- Dietary Assessments: Medical professionals can evaluate patients’ food and offer dietary advice based on protein content information.
- Research on Health Conditions: It is employed in investigations into diseases like diabetes, obesity, and malnutrition.
REFERENCES
- Dairy Knowledge. (n.d.). Dairy Farming Guide: Feeding Management of Dairy Cattle.
- Sharma, N., & Kumari, N. (2018). Evaluation of Different Forage Crops for Sustainable Livestock Production in the Central Himalayas. Indian Journal of Animal Sciences, 88(1), 118–121.
- Indira Gandhi National Open University. (n.d.). Experiment 2: Feeding Management of Dairy Animals.
- University of Wisconsin-Madison. (2015). Forage Protein and Amino Acid Requirements for Dairy Cattle.
- Central University of Technology, Mysore. (2020). Experiment 2: Feeding Management in Dairy Farming.