Chimeric antigen receptor (CAR) T-cell therapy has revolutionized cancer treatment by harnessing the immune system’s inherent ability to specifically target and destroy malignant cells. This personalized therapeutic approach involves genetically engineering a patient’s own T cells to express synthetic receptors—CARs—that recognize tumor-specific antigens independently of the major histocompatibility complex (MHC). Central to this technology is the CAR-T vector plasmid: a synthetic DNA construct that encodes the CAR protein and contains all the necessary regulatory elements to ensure proper expression and functionality within T cells.
Understanding the structural components of the CAR-T vector plasmid is essential not only for optimizing therapeutic potency and safety but also for advancing the field of cellular immunotherapy. This article provides a detailed exploration of the CAR structure itself and the essential vector elements housed within the plasmid that enable efficient CAR expression, selection, and function.
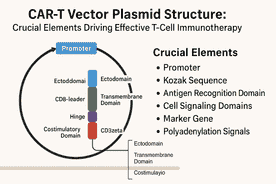
1. The CAR Protein: Modular Structure and Function
At the core of CAR-T therapy lies the chimeric antigen receptor, a synthetic transmembrane protein that equips T cells with the ability to recognize specific tumor-associated antigens and initiate cytotoxic immune responses. CARs are modular, generally composed of three key domains:
Ectodomain (extracellular antigen recognition)
Transmembrane domain (membrane anchoring)
Endodomain (intracellular signaling and activation)
Each domain is carefully engineered to maximize T cell specificity, activation, and persistence.
1.1 Ectodomain: Targeting Tumor Antigens with Precision
The ectodomain protrudes from the T cell surface and mediates antigen recognition. It contains three important subcomponents:
A) Signal Peptide
The signal peptide, often derived from the CD8α leader sequence, is a short amino acid sequence at the N-terminus that directs the nascent CAR protein to the endoplasmic reticulum. This facilitates proper folding, glycosylation, and transport to the cell surface. Without an effective signal peptide, CAR proteins may fail to reach the membrane, rendering the therapy ineffective.
B) Antigen-Binding Domain (scFv)
This domain confers target specificity. It is commonly a single-chain variable fragment (scFv), which is a fusion of the variable regions of a monoclonal antibody’s heavy (VH) and light (VL) chains linked by a flexible peptide. The scFv binds to tumor antigens directly on the cell surface, independent of MHC presentation, allowing CAR-T cells to bypass tumor immune evasion mechanisms that downregulate MHC molecules.
The affinity and specificity of the scFv critically influence therapeutic outcomes. While high affinity can improve target recognition, overly strong binding may cause activation-induced cell death or off-target toxicity. Furthermore, the epitope location on the antigen and antigen density on tumor cells also affect CAR-T efficacy. Notably, scFv domains are prone to aggregation, which can cause tonic (antigen-independent) signaling leading to premature T cell exhaustion. Engineering stable, well-folded scFvs is thus an ongoing area of research.
C) Hinge or Spacer Region
The hinge region connects the scFv to the transmembrane domain and provides structural flexibility, allowing the antigen-binding domain to reach and engage target epitopes that may be sterically hindered. The spacer length and composition significantly influence CAR function.
Commonly used spacers include hinges from CD8α or CD28, or IgG Fc regions (e.g., IgG1 or IgG4). IgG-derived spacers must often be mutated to prevent interaction with Fc gamma receptors (FcγRs) on innate immune cells, which can trigger unintended immune responses and CAR-T cell clearance. Spacer length must be tailored to the specific antigen epitope; longer spacers facilitate access to membrane-proximal epitopes but may increase tonic signaling and activation-induced cell death, whereas shorter spacers are more suitable for membrane-distal epitopes.
Besides functional roles, spacers can also serve as epitopes for antibody-based CAR detection or purification techniques, such as incorporating Strep-tag sequences.
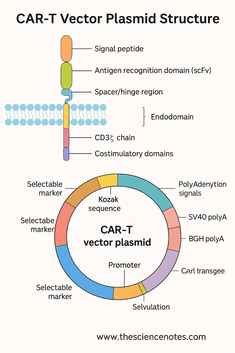
1.2 Transmembrane Domain: Anchoring and Stabilization
The transmembrane domain anchors the CAR in the T cell membrane and physically connects the extracellular recognition domain to the intracellular signaling modules. Despite its small size, this domain affects CAR expression levels, receptor stability, and signaling.
Transmembrane domains are often derived from native T cell proteins such as:
CD3ζ (CD247)
CD4
CD8α
CD28
The choice of transmembrane domain can influence how the CAR integrates with endogenous T-cell receptor (TCR) complexes. For instance, CD3ζ transmembrane domains can promote CAR incorporation into native TCR complexes, potentially enhancing activation but possibly destabilizing receptor complexes. CD8α and CD28 transmembrane domains tend to confer greater receptor stability and consistent surface expression. Additionally, transmembrane domains can affect cytokine release profiles and tonic signaling, factors closely tied to safety and efficacy.
1.3 Intracellular Signaling Domains: Initiating T Cell Activation
The intracellular endodomain translates antigen recognition into T cell activation and cytotoxic response. It contains signaling motifs that trigger proliferation, cytokine release, and target cell killing.
A) CD3ζ Chain
The CD3ζ chain is the primary signaling domain in all CAR designs. It contains three immunoreceptor tyrosine-based activation motifs (ITAMs) that, when phosphorylated upon antigen binding, initiate downstream signaling cascades essential for T cell activation.
B) Costimulatory Domains
To enhance T cell function, persistence, and prevent exhaustion, CARs incorporate one or more costimulatory domains upstream of CD3ζ:
CD28: Promotes rapid T cell expansion and potent cytokine secretion but may lead to shorter persistence.
4-1BB (CD137): Enhances T cell survival and memory formation, contributing to sustained activity.
Others: ICOS, CD27, OX40, and combinations such as MYD88 plus CD40 are under investigation for optimized signaling.
CAR generations are classified based on their intracellular domain complexity:
First-generation CARs: CD3ζ alone.
Second-generation CARs: CD3ζ plus one costimulatory domain (most FDA-approved CARs belong here).
Third-generation CARs: CD3ζ plus two costimulatory domains for potentially enhanced function.
2. The CAR-T Vector Plasmid: Essential Genetic Elements
The CAR transgene is embedded within a plasmid vector designed to maximize expression, stability, and selection capability in T cells and during plasmid propagation.
2.1 Promoters: Driving CAR Expression
Efficient transcription of the CAR gene depends on the choice of promoter controlling the expression cassette. Common promoters include:
EF1α (Elongation Factor 1 alpha): A strong, constitutive promoter active in a broad range of cell types including T cells. It enables stable, high-level expression without silencing.
CMV (Cytomegalovirus) immediate early promoter: A viral promoter with robust early expression but can be silenced in certain primary cells over time.
PGK (Phosphoglycerate kinase) promoter: A weaker, constitutive promoter used when moderate expression is desired to reduce tonic signaling or toxicity.
The promoter choice balances the need for sufficient CAR protein on the surface with minimizing risks of overactivation or exhaustion.
2.2 Kozak Sequence: Optimizing Translation Initiation
Immediately upstream of the start codon (AUG), the Kozak consensus sequence (GCCGCCACC) enhances ribosome binding and initiates efficient translation of the CAR mRNA. Incorporation of an optimized Kozak sequence is standard practice to maximize protein yield.
2.3 Polyadenylation Signals: Ensuring mRNA Stability
Following the CAR coding sequence, polyadenylation (polyA) signals promote proper transcription termination, mRNA stability, and nuclear export. Two commonly used polyA sequences are:
SV40 late polyA: From simian virus 40, widely used in mammalian expression vectors.
BGH polyA: Bovine growth hormone polyadenylation sequence, equally effective at stabilizing transcripts.
Proper polyadenylation reduces degradation and enhances CAR protein production.
2.4 Selectable Markers and Reporter Genes
To identify and select successfully transfected or transduced T cells, plasmids often include markers such as:
Antibiotic resistance genes (e.g., neomycin resistance): Allow for antibiotic selection during cell culture.
Fluorescent proteins (e.g., EGFP, mCherry): Enable visualization and sorting via flow cytometry.
Dual markers: Combining antibiotic resistance and fluorescence for flexible selection.
These elements are typically controlled by separate promoters and do not interfere with CAR expression.
2.5 Origin of Replication (ori)
For plasmid propagation in bacteria, an origin of replication such as pUC ori is included. This allows high-copy replication in E. coli, facilitating large-scale plasmid production necessary for clinical and research applications.
2.6 Bacterial Selection Marker
The ampicillin resistance gene (Amp^R) is used for antibiotic selection in bacterial culture, ensuring only bacteria harboring the plasmid survive.
3. FDA-Approved CAR-T Products: Real-World Vector Examples
Several CAR-T cell therapies have received FDA approval, each using different but fundamentally similar vector designs.
| Trade Name | Generic Name | Company | Approval Date | Target Antigen | Indications |
|---|---|---|---|---|---|
| KYMRIAH™ | Tisagenlecleucel | Novartis | Aug 2017 | CD19 | Relapsed/Refractory B-ALL |
| YESCARTA™ | Axicabtagene ciloleucel | Gilead/Kite | Oct 2017 | CD19 | Relapsed/Refractory DLBCL |
| TECARTUS™ | Brexucabtagene autoleucel | Gilead/Kite | Jul 2020 | CD19 | Relapsed/Refractory Mantle Cell Lymphoma |
| BREYANZI® | Lisocabtagene maraleucel | Bristol Myers Squibb | Feb 2021 | CD19 | Relapsed/Refractory Large B-cell Lymphoma |
| ABECMA® | Idecabtagene vicleucel | Bristol Myers Squibb | Mar 2021 | BCMA | Relapsed/Refractory Multiple Myeloma |
| CARVYKTI® | Ciltacabtagene autoleucel | Janssen | Feb 2022 | BCMA | Relapsed/Refractory Multiple Myeloma |
These products exemplify how modular CAR and vector designs are adapted to target different antigens and diseases while maintaining a core plasmid backbone architecture.
https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
4. Concluding Remarks: The Future of CAR-T Vector Design
The CAR-T vector plasmid is far more than a simple DNA delivery vehicle; it is a complex, finely tuned genetic tool essential for successful CAR-T cell therapy. Each component—from the scFv’s affinity and spacer length, through the choice of transmembrane and signaling domains, to promoter strength and selection markers—affects the therapeutic outcome.
While first- and second-generation CARs have achieved remarkable clinical successes, ongoing challenges include antigen escape, T cell exhaustion, and toxicity. These issues highlight the need for improved vector designs, including inducible promoters, novel costimulatory domains, optimized scFvs, and safety switches.
As CAR-T therapies expand beyond hematological malignancies into solid tumors and other diseases, vector plasmids will need to evolve with advanced regulatory elements and finely tuned CAR architectures to meet new challenges. Enhanced high-throughput screening and computational design tools will accelerate this development, ultimately improving efficacy, safety, and accessibility for patients worldwide.