Definition of Acid Value
The amount of free fatty acids that are present in a substance typically a fat or oil is measured by its acid value and called as acid value in fats and oils. It is expressed as the amount of potassium hydroxide (KOH) required to neutralize the free fatty acids present in one gram of the substance.
- Oils and fats used in a variety of industries, including food, cosmetics, and pharmaceuticals, are evaluated for their quality and purity using a measure known as the acid value.
- Considering that the presence of free fatty acids is an indication of oxidation, it can also be used to determine the level of rancidity in oils and fats.
- Since free fatty acids are often produced during the breakdown of triglycerides, it is a relative indicator of rancidity. Additionally, the value is given as a percentage of the free fatty acids oleic, lauric, ricinoleic, and palmitic acids.

Principle of Determination of Acid Value in Fats and Oils
- The amount of carboxylic acid groups in an organic compound, like a fatty acid, can be measured by the acid number. A common method involves titrating a known quantity of sample that has been dissolved in an organic solvent with a potassium hydroxide solution that has a known concentration and uses phenolphthalein as a color indicator.
- By dissolving a known amount of fat or oil in pure alcohol and titrating the result against a standard KOH solution while using phenolphthalein as an indicator, it can be calculated.
- The number of free acids contained in a specific fat or oil can be determined by the acid value.
- A high acid value implies that the given sample of oil is an old one which has gone rancid.
Procedure for determining Acid value in Fats and Oils
Apparatus required
- Balance machine
- Burette with stand
- Measuring cylinder
- Conical flask
- Hot plate
Procedure of Determination of Acid Value in Fats and Oils
1. Chemical preparation
- Phenolphthalein indicator solution: Dissolve 2 gram of phenolphthalein in 100mL of ethyl alcohol and mix well by shaking.
- 0.1N Sodium hydroxide solution preparation: Dissolve 4g sodium hydroxide pellets into 900ml of distilled water, cool and makes a final volume of 1000ml. standardize the solution before use.
2. Sample preparation
- Take 10g of oil sample in a conical flask and note the sample weight.
- Measure 50ml ethanol (99%) and pour into another conical flask(150ml).
- Add 2-3 drops of phenolphthalein indicator solution and shake and neutralize by adding 0.1N NaOH until the light pink colour solution is formed.
- Now, the neutralized ethanol is added to the flask containing sample and shake the flask to mix the solution.
- Boil the mixture by placing the flask on hot plate until the sample dissolve in ethanol completely.
- Continue the boiling until the sample is dissolved completely.
- Shaking with hands should be continued periodically.
3. Titration
- Take0.1N NaOH (standardized) in a burette.
- Note the initial burette reading.
- Start titration by adding few drops of phenolphthalein indicator.
- Titration should be carried out with vigorous agitation of the flask to get accurate result.
- Stop the titration when the solution colour is changed into a white pink.
- Note the final burette reading.
4. Calculation
Acid value =
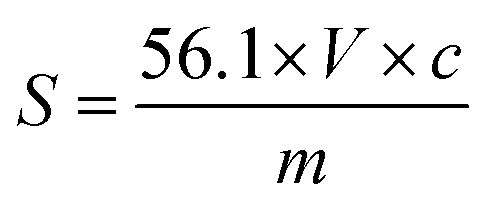
where,
- MW is molecular weight of NaOH
- N is a normality of NaOH
- V(C) is a volume of NaOH
- WS is a sample weight

Importance of Acid value determination in fats and oils
The amount of free fatty acids present in oils and fats can be measured by the acid value. It is an important parameter used in the analysis and quality control of these substances. The acid value of fats and oils can be used for several applications , some of which are:
Determination of freshness: The amount of triglyceride hydrolysis in oils and fats is determined by the acid value. As oils and fats age or undergo rancidity, the free fatty acids increase, leading to an increase in the acid value. In order to assess the freshness and shelf life of oils and fats, the acid value can be used.
Quality control: The acid value is used as a quality control parameter for oils and fats. Different types of oils and fats have different acceptable levels of acid value. For example, crude palm oil’s acid value can range up to 5 mg KOH/g, whereas extra virgin olive oil’s acid value should be less than 0.8 mg KOH/g. The quality of oils and fats can be determined by measuring the acid value and then compared to the acceptable range.
Identification of adulteration: The presence of adulterants in oils and fats can also be determined using the acid value. Adulteration with cheaper oils or fats can increase the acid value beyond the acceptable limit, thereby indicating adulteration.
Learn more:
https://www.linkedin.com/in/binodgc/