Muscles are composed of specialized proteins that enable them to contract and produce movement. Among these proteins, actin and myosin play crucial roles in muscle contraction. They work together to facilitate both voluntary and involuntary movements in humans and other animals. Actin and myosin are integral to the function of muscle cells and are also involved in various cellular processes. This note explores the differences between actin and myosin, highlighting their roles, structures, and functions in muscle contraction.
Actin
Definition and Structure
Actin is a globular protein, known for forming thin filaments in muscle cells. In its monomeric form, it is referred to as G-actin (globular actin). When polymerized, G-actin forms filamentous actin (F-actin), which is a major component of the cytoskeleton in eukaryotic cells. Actin filaments are crucial for maintaining cell shape, enabling cell motility, and facilitating various cellular processes like division and intracellular transport.
Actin filaments are approximately 7 nanometers in diameter and vary in length, typically ranging from 2 to 2.6 micrometers in muscle cells. The filaments are composed of two intertwined strands of actin molecules, giving them a helical structure.
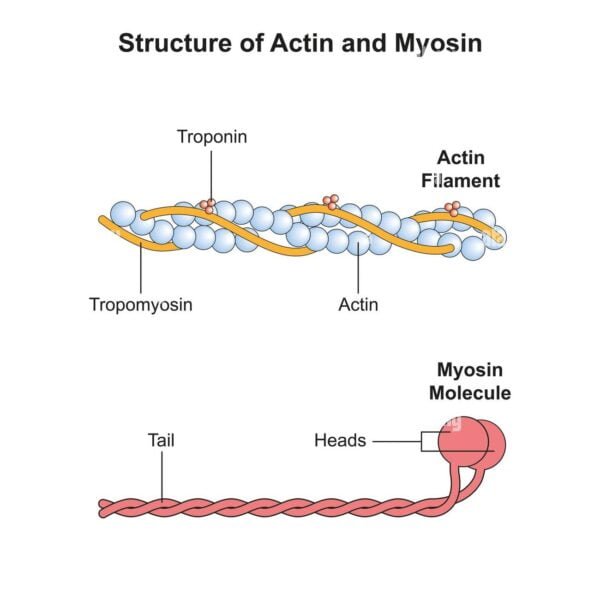
Regulatory Proteins
Actin filaments are associated with several regulatory proteins, including tropomyosin and troponin. Tropomyosin binds along the groove of the actin filament and blocks the myosin-binding sites, while troponin, which binds to tropomyosin, regulates the interaction between actin and myosin. The binding of calcium ions to troponin causes a conformational change that moves tropomyosin away from the binding sites, allowing myosin to bind to actin and initiate contraction.
Location
In muscle cells, actin filaments are present in both the A (anisotropic) and I (isotropic) bands of the sarcomere. The I band contains only actin filaments, while the A band contains both actin and myosin filaments. Actin filaments are anchored to the Z-disc at the ends of the sarcomere.
Appearance and Surface
Actin filaments have a smooth surface. They appear lighter in striation patterns when observed under a microscope compared to myosin filaments. The smoothness of the actin surface is due to the consistent arrangement of G-actin subunits along the filament.
Function
Actin filaments primarily function in maintaining cell shape, facilitating movement, and interacting with myosin to produce muscle contractions. During contraction, actin filaments slide into the H-zone of the sarcomere, which is the central region of the A band.
Abundance
Actin filaments are more numerous than myosin filaments within muscle cells. For every myosin filament, there are approximately six actin filaments, reflecting their relative abundance.
Myosin
Definition and Structure
Myosin is a motor protein characterized by its ability to convert chemical energy from ATP hydrolysis into mechanical energy. It forms thick filaments in muscle cells and plays a central role in muscle contraction. Myosin proteins are composed of heavy chains and light chains, with the heavy chains forming long, rod-like tails and globular heads that interact with actin filaments.
Myosin filaments are thicker and longer than actin filaments, measuring about 15 nanometers in diameter and 4 to 5 micrometers in length. The filaments are composed of multiple myosin molecules organized into a bipolar structure, with the heads projecting outward.
Regulatory Proteins
Myosin filaments are associated with meromyosin, which includes the light chains and the head domains that interact with actin. The head regions of myosin contain ATPase activity, which is crucial for the conversion of ATP to mechanical energy.
Location
Myosin filaments are predominantly found in the A bands of the sarcomere. They overlap with actin filaments in the A band but are not present in the I band.
Appearance and Surface
Myosin filaments have a rough surface due to the presence of protruding myosin heads. They appear darker in striation patterns compared to actin filaments, contributing to the A band’s darker appearance.
Function
Myosin filaments do not slide into the H-zone during contraction. Instead, they form cross-bridges with actin filaments, facilitating the sliding of actin filaments towards the center of the sarcomere. This sliding mechanism is fundamental to muscle contraction.
Abundance
Myosin filaments are less abundant than actin filaments. Typically, one myosin filament interacts with multiple actin filaments, reflecting their lower number in muscle cells.
Mechanism of Muscle Contraction
Muscle contraction is facilitated by the sliding filament model, which describes how actin and myosin interact to produce movement. When a muscle is stimulated by a motor neuron, calcium ions are released from the sarcoplasmic reticulum. These ions bind to troponin, causing a shift in tropomyosin that exposes the binding sites on actin.
Myosin heads, which are energized by the hydrolysis of ATP, bind to the exposed sites on actin to form cross-bridges. The myosin heads then pivot, pulling the actin filaments toward the center of the sarcomere. This action shortens the sarcomere, resulting in muscle contraction. The process is repeated as long as ATP and calcium ions are available.
Key Differences Between Actin and Myosin
| Aspect | Actin | Myosin |
| Definition and Function | Globular protein forming thin filaments; involved in muscle contraction, cell shape, motility, and division. | Motor protein; converts ATP hydrolysis into mechanical energy; forms thick filaments and interacts with actin for muscle contraction. |
| Structure | Thin filaments, approximately 7 nm in diameter; helical structure made of G-actin. | Thick filaments, approximately 15 nm in diameter; consists of long, rod-like tail and globular heads. |
| Size | Short (2-2.6 µm in length), thin (0.005 µm in diameter). | Long (4-5 µm in length), thick (0.01 µm in diameter). |
| Surface Characteristics | Smooth surface. | Rough surface due to protruding myosin heads. |
| Regulatory Proteins | Tropomyosin (blocks myosin-binding sites); Troponin (binds calcium and regulates tropomyosin position). | Meromyosin (includes the head and tail domains, involved in cross-bridge formation). |
| Location in the Sarcomere | Found in both A and I bands; anchored to Z-discs. | Primarily found in A bands; anchored at M-line. |
| Abundance | More abundant; typically six actin filaments per myosin filament. | Less abundant; one myosin filament per six actin filaments. |
| Cross-Bridge Formation | Does not form cross-bridges directly; provides binding sites for myosin. | Forms cross-bridges with actin filaments during contraction. |
| Association with ATP | Not directly associated with ATP. | Directly associated with ATP; ATPase activity drives movement. |
| Sliding Mechanism During Contraction | Slides into the H-zone during contraction. | Stays stationary while pulling actin filaments towards the center of the sarcomere. |
| Ends and Binding | One end free (barbed or plus end), other end bound to Z-disc (pointed or minus end). | Both ends free; heads remain associated with ATP. |
| Appearance Under Microscopy | Appears as lighter striations (I bands). | Appears as darker striations (A bands). |
| Additional Roles | Forms microfilaments in cytoskeleton; involved in cell division and motility. | Functions as a molecular motor in muscle contraction and other cellular processes depending on the type of myosin. |
Conclusion
Actin and myosin are fundamental to muscle function and various cellular processes. Actin forms thin filaments that provide structural support and interact with myosin during contraction. Myosin, as a motor protein, forms thick filaments and is essential for generating the force necessary for muscle movement. Understanding the differences between these two proteins highlights their distinct roles in muscle physiology and their cooperative function in the mechanism of muscle contraction.