Author: Binod GC
E-cadherin is a type-1 cadherin which is considered as an ideal of all cadherin in normal as well as in aberrant conditions. It is a calcium dependent cell-cell adhesion molecule with major role in epithelial cell conformity. E-cadherin has binding sites for Ca2+ in its extracellular domain which extends from cell surface to bind to cadherin on adjacent cells by lateral dimerization (Shapiro et al. 1995). This allows cell-cell adhesion. E-cadherin, via its cytoplasmic domain, binds to β-catenin and p-120 catenin (members of the catenin family). Binding to β -catenin and p-120 catenin link the multi protein complex to the actin cytoskleleton through α-catenin. The cytoplasmic domain consists of catenin-binding domain (CBD) and juxtamembrane binding domain (JMD). Adhesive strength is contributed by JMD via p120-catenin. The CBD interacts with β-catenin and ϒ-catenin. Likewise, α-catenin links the bound β-catenin to the actin cytoskeleton. This stabilizes the cell-cell adhesion (Kemler 1993).
In epithelial tissues, E cadherin is a key mediator of cell-cell adhesion and loss of E-cadherin can promote invasiveness and metastatic behavior in many epithelial tumors (Behrens et al. 1993). In the absence of E-cadherin, there is lack of functional tight junction and desmosome formation emphasizing its central role in the regulation of epithelial cell-cell contacts (Tunggal et al. 2005). Formation of E-cadherin trans-homodimers regulates cell-cell adhesion. The E-cadherin molecule contains an ectodomain composed of 5 extracellular cadherin (EC1-EC5). E-cadherin as a pro-protein is transported from the golgi complex to the cell surface. E-cadherin associates early with β-catenin during biosynthesis and after removal of pro-domain this complex reach the cell surface where p120-catenin and α-catenin associate (Van Roy et al. 2008).
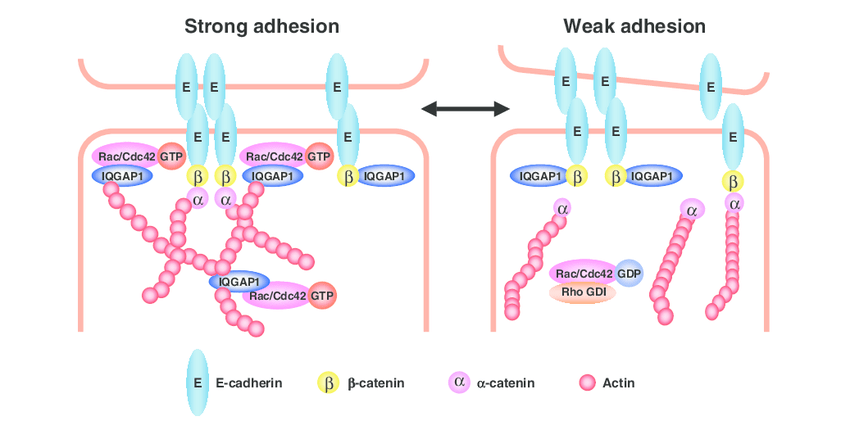
E-cadherin acts on the maintenance of an epithelial barrier, removing different harmful agents away from the tissue. E-cadherin modulates the transepithelial passage of innate and adaptive immune cells. Innate immune functions such as the release of cytokines and chemokines can be regulated by E-cadherin (Nawijn et al. 2011). In addition to this, E-cadherin also has cell signaling function.
E-cadherin can interact with the inhibitory killer cell lectin-like receptor G1 (KLRG1) (Ito et al. 2006). After KLRG1 recognizes the N-terminal homodimeric interface of E-cadherin EC1, then only KLRG1 can bind to the monomeric form of E-cadherin (Li et al. 2009). If KLRG1 and TCR/CD3 are coengaged, KLRG1 signaling may result in defective Akt phosphorylation which can lead to proliferative dysfunction (Henson et al. 2009).
E-cadherin has also pivotal role in Wnt and cadherin pathway. β -catenin activities are linked to Wnt and cadherin pathway (Nelson et al. 2004). β-catenin gets phosphorylated in the absence of Wnt ligand binding to its receptor which targets β-catenin for ubiquitination and subsequent proteasomal degradation. However, once Wnt signal is activated by the ligand, β-catenin phosphorylation is inhibited and causes the accumulation of free β-catenin and its nuclear import. Accumulation of active β-catenin/transcription factor complex results in the expression of Wnt regulated genes (Staal et al. 2008). E-cadherins and adherens junction control the cytoplasmic level of β-catenin. Interfering this junction will negatively affect the β-catenin level and will thus affect the Wnt pathway. Serine/threonine phosphorylation status determines the stability of adherens junction and E-cadherin/β-catenin complex. Serine/threonine phosphorylation of β-catenin and E-cadherin increases stability of cadherin catenin complex whereas tyrosine phosphorylation can disrupt the complex (Taddei et al. 2002, Behrens et al. 1993).
In advanced tumors, E-cadherin expression is observed to be decreased which is related with a higher incidence of metastasis (Birchmeier et al. 1994). Genetic as well as epigenetic alteration of E-cadherin encoding gene (CDH1) can have a significant impact on a metastatic spread. Different studies have revealed that reduced expression of E-cadherin is due to loss of heterozygosity, mutation and hyper methylation in CpG-island of CDH1 gene promoter by specific transcription factor in various epithelial tumors (Zhai et al. 2008).
The depletion of E-cadherin and its effect in loosening cell-cell adhesion is initial stage of epithelial-mesenchymal transition (EMT) process (Kalluri et al. 2009). EMT is regarded as potentially important event in the metastatic spread of tumor cells resulted by the destabilization of cell-cell adhesion and acquiring invasive mesenchymal phenotype (Guarino et al. 2007). EMT is not only related to cancer, but it is also observed in normal cell behavior. EMT is needed for process like embryogenesis, organ development and wound repair which is regulated by similar signaling to those control cancer EMT (Thiery 2002). Even though cancer cells undergo EMT for moving away from primary tumor and cell invasion can occur with intact and functional cell-cell adhesion, a limited loosening of cell-cell contact can be sufficient for invasion (Christiansen et al. 2006).
The role of E-cadherin as a tumor suppressor is well known. Surprisingly, some recently published reports support its role as oncogene acting through alternate pathways. For instance, ovarian cancer shows a more epithelial phenotype in early tumor progression (Gallo et al. 2010). E-cadherin is highly expressed in ovarian cancer but rare in normal ovarian tissues (Sundfeldt et al. 1997).
In conclusion, depletion in E-cadherin results in nonfunctional cell-cell adhesion system which evokes cancer metastasis and invasion. The primary role of E-cadherin is to control EMT. Various studies suggest that E-cadherin is a potential tumor suppressor gene. E-cadherin’s role in wnt signaling implies that it has major role in migration, proliferation and apoptosis.
References:
- Shapiro L, Fannon AM, Kwong PD, et al (1995). Structural basis of cell-cell adhesion by cadherins. Nature; 374:327–37.
- Kemler R (1993). From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet; 9:317–21.
- Behrens J, Vakaet l, Friis R,Winterhager E, Van Roy F, Mareel M. M. and Birchmeier W (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120, 757–766.doi:10.1083/jcb.120.3.757
- Tunggal JA, Helfrich I, Schmitz A, et al (2005). E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J;24(6):1146-1156.
- Van Roy F, Berx G (2008). The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci;65(23):3756-3788.
- Nawijn MC, Hackett TL, Postma DS, van Oosterhout AJM, Heijink IH (2011). E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol;32(6):248-255.
- Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N (2006). Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med;203(2):289-295.
- Li Y, Hofmann M, Wang Q, et al (2009). Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing-self recognition. Immunity;31(1):35-46.
- Henson SM, Franzese O, Macauley R, et al (2009).KLRG1 signaling induces defective Akt (Ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood;113(26):6619-6628.
- Nelson WJ, Nusse R (2004). Convergence of Wnt, ß-catenin, and cadherin pathways. Science;303:1483–7.
- Staal FJ, Luis TC, Tiemessen MM (2008). WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol;8(8):581-593.
- Taddei ML, Chiarugi P, Cirri P, et al (2002). Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res;62:6489–99.
- Birchmeier W. and Behrens J. (1994). Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 1198, 11–26.
- Zhai B, Yan HX, Liu SQ, et al (2008). Reduced expression of E-cadherin/catenin complex in hepatocellular carcinomas. World J Gastroenterol;14:5665–73
- Kalluri R. and Weinberg R. A. (2009). The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428. doi:10.1172/JCI39104
- Guarino M., Rubino B. and Ballabio G. (2007). The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39, 305–318.doi:10.1080/00313020701329914
- Thiery JP (2002). Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454. doi:10.1038/nrc822
- Christiansen J. J. and Rajasekaran A. K (2006). Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 66, 8319–8326. doi:10.1158/0008-5472.CAN-06-0410
- Gallo D, Ferlini C, Scambia G (2010). The epithelial-mesenchymal transition and the estrogen-signaling in ovarian cancer. Curr Drug Targets;11:474–81.
- Sundfeldt K, Piontkewitz Y, Ivarsson K, et al (1997). E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Cancer;74:275–80.