Electrons are negatively charged subatomic particles that play a central role in the structure of atoms and the behavior of matter. Although electrons have very little mass compared to protons and neutrons, they occupy most of an atom’s volume and determine how atoms interact with one another. From chemical bonding to the properties of elements, electrons are essential to understanding chemistry and atomic structure.
Electrons are attracted to the positively charged nucleus of an atom and exist in specific regions associated with defined energy levels. These regions are organized into shells, sub-shells, and orbitals, each describing the energy, position, and probability of finding an electron.
What Are Electrons?
An electron is a subatomic particle with a negative electrical charge of –1. Unlike protons and neutrons, which are found in the nucleus, electrons move around the nucleus in regions of space called electron clouds.
Although electrons have negligible mass—about 1/2000 the mass of a proton—they are critically important. Their arrangement determines whether an atom is stable, how it reacts with other atoms, and what chemical bonds it can form.
Electrons Orbit the Nucleus
Electrons are found outside the nucleus in discrete regions associated with energy levels, often referred to as electron shells.
Electron Shells and Energy Levels
Electrons closer to the nucleus have lower energy
Electrons farther from the nucleus have higher energy
As distance from the nucleus increases, the energy of the electron increases
The innermost shell can hold only a small number of electrons, while outer shells have more space and can hold more electrons. This structure explains why atoms can have many electrons without collapsing inward.
Sub-Shells and Orbitals
Electron shells are further divided into sub-shells, which describe energy levels more precisely. Each sub-shell contains one or more orbitals.
What Is an Orbital?
An orbital is not a fixed path like a planet’s orbit. Instead, it is a region of probability where an electron is most likely to be found. Orbitals come in different shapes and orientations, reflecting the complex behavior of electrons.
Together, shells, sub-shells, and orbitals create a structured system that governs how electrons are distributed around the nucleus.
Electron Energy and Distance from the Nucleus
The energy of an electron is directly related to its distance from the nucleus:
Low energy electrons are found closer to the nucleus
High energy electrons occupy outer shells
Outer shells have more room and can hold more electrons
Because of this arrangement, electrons fill the lowest available energy levels first before occupying higher ones. This pattern is essential for understanding atomic stability and reactivity.
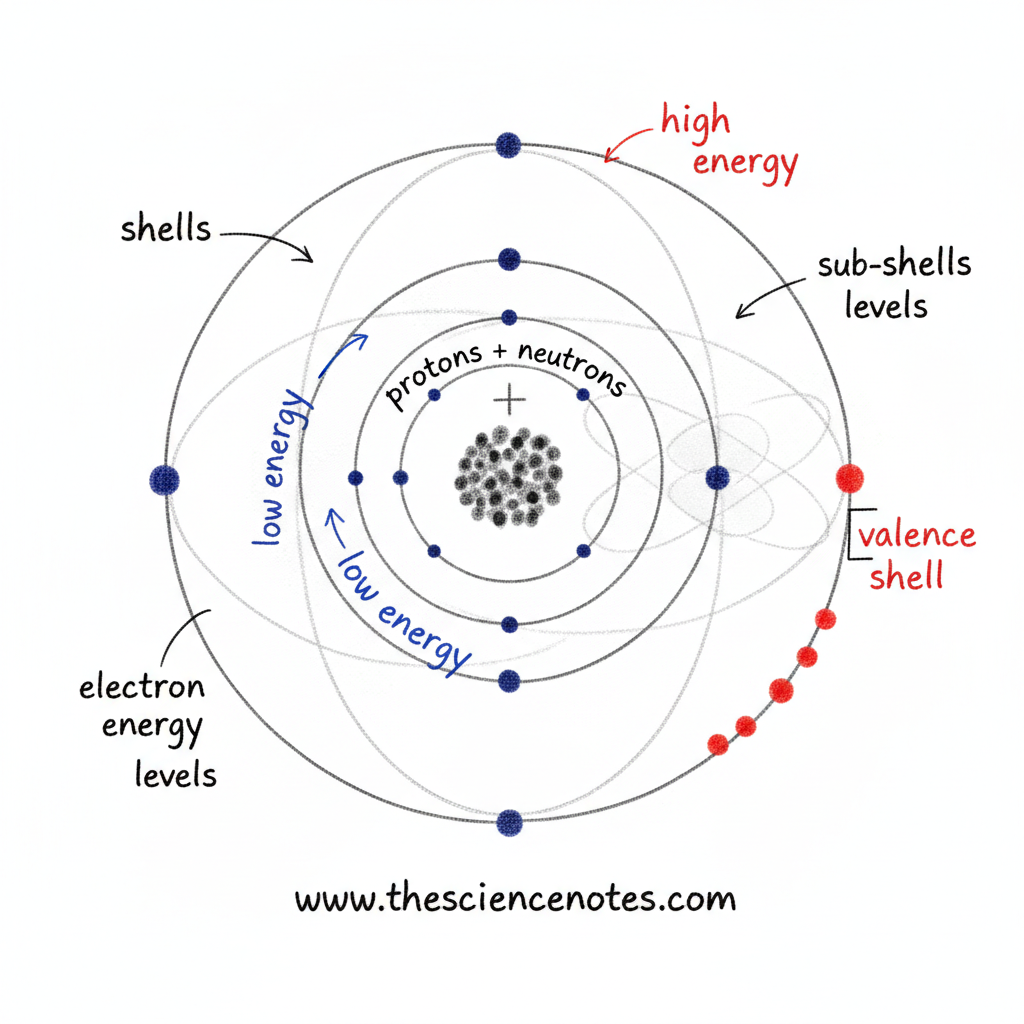
Valence Electrons and Chemical Properties
The electrons in the outermost shell of an atom are called valence electrons. These electrons are especially important because they are involved in chemical bonding.
Why Valence Electrons Matter
Valence electrons determine:
The reactivity of an element
The types of chemical bonds it can form
The physical and chemical properties of the element
Atoms tend to gain, lose, or share valence electrons in order to achieve a more stable electron configuration.
Ionic and Covalent Bonds
Valence electrons allow atoms to form bonds in two main ways:
Ionic Bonds
Form when electrons are transferred from one atom to another
One atom becomes positively charged, the other negatively charged
Common in salts and ionic compounds
Covalent Bonds
Form when electrons are shared between atoms
Creates strong bonds in molecules like water and carbon dioxide
These bonding behaviors explain how atoms combine to form the vast variety of substances found in nature.
Discovering the Electron
The electron was the first subatomic particle to be discovered, marking a major turning point in atomic theory.
J. J. Thomson and Cathode Ray Tubes
In the late 1890s, physicist J. J. Thomson conducted experiments using cathode ray tubes—glass tubes with electrodes connected to a power source.
When electricity was applied:
A beam of particles traveled from the negative electrode (cathode) to the positive electrode (anode)
A phosphor-coated screen glowed when struck by the beam
This beam was known as a cathode ray.
Evidence of Negative Charge
Thomson passed the cathode ray between two charged metal plates:
One positively charged
One negatively charged
The ray bent toward the positively charged plate and away from the negatively charged one. Since opposite charges attract and like charges repel, this demonstrated that the particles in the ray carried a negative charge.
Measuring Electron Mass
Further experiments allowed Thomson to calculate the mass-to-charge ratio of the cathode ray particles. The results showed that these particles were extremely light—about 1/2000 the mass of the smallest known atom.
From this, Thomson concluded:
These particles were universal components of atoms
Atoms must contain many electrons
Later discoveries of protons and neutrons explained how atoms could contain negatively charged electrons while remaining electrically neutral overall.
Electrons and Atomic Volume
Although electrons are tiny, they occupy most of an atom’s volume. The electron cloud surrounding the nucleus is mostly empty space, which explains why atoms are not solid in the way they appear at a macroscopic scale.
Electrons remain near the nucleus due to the electrical attraction between their negative charge and the positive charge of protons.
Why Electrons Are Essential
Electrons are responsible for:
Chemical bonding
Electrical conductivity
The properties of elements
The formation of molecules
Energy transfer in chemical reactions
Without electrons, atoms could not interact, molecules could not form, and matter as we know it would not exist.
Conclusion
Electrons are fundamental to atomic structure and chemical behavior. Their negative charge, organization into energy levels, and role as valence electrons explain how atoms bond and why elements behave differently from one another.
From their discovery in cathode ray tubes to their central role in modern chemistry, electrons have reshaped our understanding of matter. Though incredibly small, they govern the structure, stability, and diversity of the material world.