Elements are the fundamental building blocks of matter. They are the smallest units that cannot be broken down into simpler substances through chemical processes. Everything around us—air, water, soil, and living organisms—is made up of combinations of these elements. Although scientists have identified 118 known elements, only a fraction are naturally occurring, and even fewer are essential for life.
Understanding how elements are organized, how they behave, and how they contribute to living systems is critical to fields such as chemistry, biology, medicine, and environmental science. At the heart of this understanding lies the periodic table, a powerful tool that reveals the physical and chemical properties of every known element.
What Are Elements and Why Are They Important?
An element is a pure substance consisting of only one type of atom. Each element is defined by its atomic number, which represents the number of protons in its nucleus. This number determines an element’s identity and its position on the periodic table.
While there are 118 known elements, only 92 occur naturally on Earth. Even more striking is the fact that humans require just 25 elements to live and reproduce. Of those, only four elements—oxygen, carbon, hydrogen, and nitrogen—make up approximately 96% of all living matter.
These elements are essential because they form the molecules that support life, including proteins, carbohydrates, lipids, and nucleic acids.
The Periodic Table: Organizing the Elements
The periodic table is a systematic arrangement of all known elements based on increasing atomic number and recurring chemical properties. Each square on the table provides critical information, including:
Atomic number
Chemical symbol
Element name
Atomic weight
For example, sodium has the chemical symbol Na, derived from its Latin name natrium. This standardized system allows scientists around the world to communicate clearly and consistently.
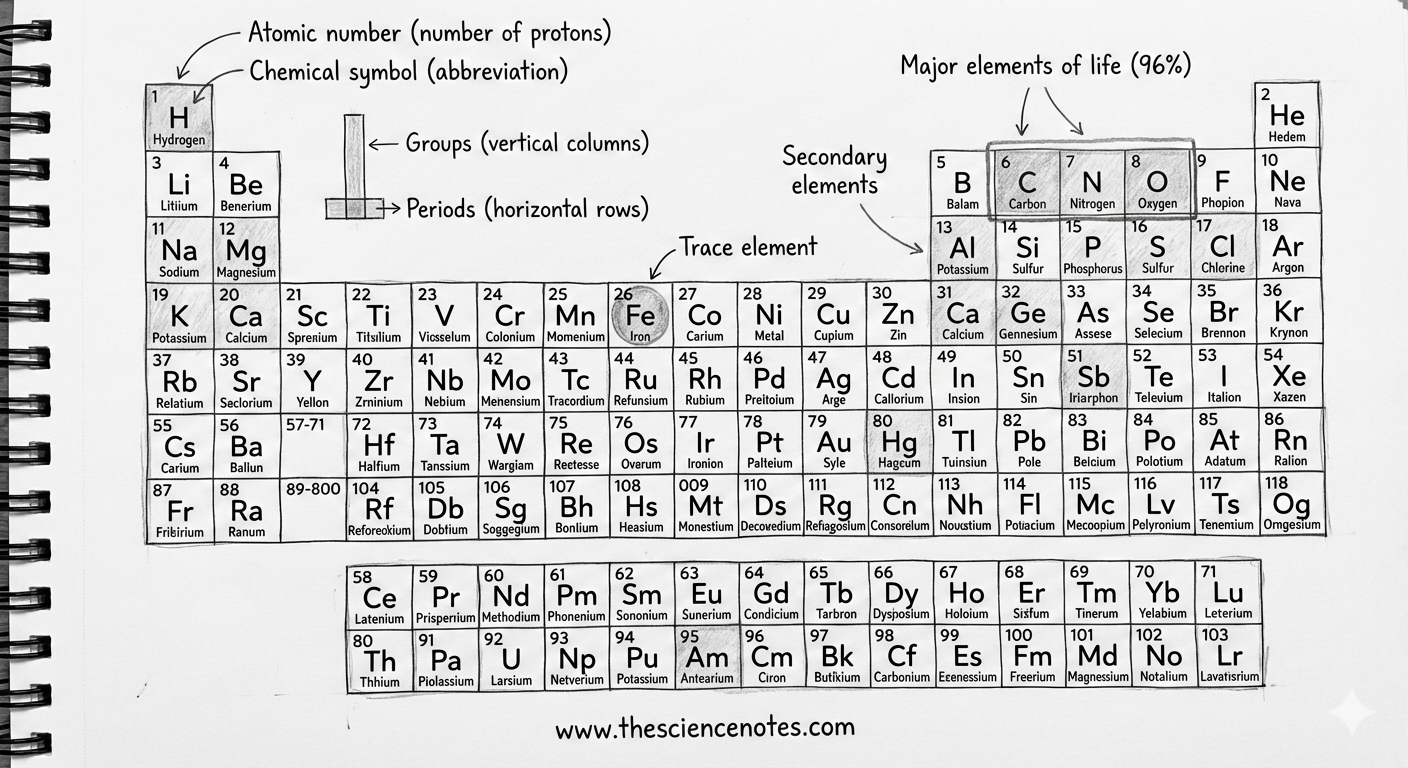
Groups and Periods: Patterns in Chemical Behavior
Beyond atomic number, the periodic table is organized into columns (groups) and rows (periods) that reflect deeper chemical relationships.
Groups (Columns)
Elements in the same group share similar chemical properties, even though their atomic sizes may differ. This similarity arises because they have the same number of electrons in their outermost shell, which largely determines how elements react and bond with others.
Periods (Rows)
Elements in the same period are more similar in size and have their electrons arranged in comparable energy levels. However, their chemical properties can vary greatly across a period as the number of protons and electrons increases.
This unique organization allows scientists to predict how elements will behave, even if they have never been studied before.
Major Elements That Make Up the Human Body
All living organisms on Earth contain oxygen, carbon, hydrogen, and nitrogen. In humans, these four elements account for 96% of total body mass.
Oxygen (O): Essential for cellular respiration and energy production
Carbon (C): The backbone of all organic molecules
Hydrogen (H): Plays a key role in energy transfer and molecular structure
Nitrogen (N): Crucial for proteins and nucleic acids like DNA
These elements form complex molecules that drive every biological process, from metabolism to reproduction.
Secondary Elements in Living Matter
The remaining 4% of the human body consists primarily of the following elements, listed in order of abundance:
Calcium (Ca): Supports bones, teeth, and muscle function
Phosphorus (P): Essential for DNA, RNA, and energy transfer (ATP)
Potassium (K): Regulates nerve signals and fluid balance
Sulfur (S): Found in certain amino acids and proteins
Sodium (Na): Helps maintain fluid balance and nerve impulses
Chlorine (Cl): Important for digestion and electrolyte balance
Magnesium (Mg): Required for enzyme function and muscle activity
Though present in smaller amounts, these elements are vital for maintaining normal physiological functions.
Trace Elements: Small Amounts, Big Impact
Trace elements are elements required by the body in extremely small quantities—less than 0.01% of total body weight—yet they are essential for health and survival.
Iron: A Critical Trace Element
One of the most important trace elements is iron (Fe). Iron plays a central role in red blood cells by helping hemoglobin bind oxygen and transport it throughout the body.
A deficiency in iron can lead to iron-deficiency anemia, a condition marked by:
Fatigue
Shortness of breath
Weakness
Irregular heart rhythms
This highlights how even minute amounts of certain elements can have profound effects on human health.
Harmful Elements and Their Effects on Living Organisms
Not all elements are beneficial. Some can be toxic or even lethal, particularly heavy metals.
Mercury and Heavy Metal Toxicity
Mercury is a heavy metal that can cause severe health problems even at low levels of exposure. Depending on the tissues affected, mercury toxicity can damage the nervous system, kidneys, and other organs. In larger doses, it can be fatal.
One of the major concerns with heavy metals is their ability to accumulate in living tissues over time, a process known as bioaccumulation.
Environmental Impact and Bioremediation
Heavy metals often enter ecosystems through industrial pollution and can move through the food web, beginning with primary producers like plants and algae and eventually affecting higher trophic levels, including humans.
Scientists are exploring bioremediation, a biological approach to removing heavy metal contaminants from the environment. This research requires a deep understanding of both:
The chemistry of the contaminants
The biology of the organisms first affected
Such efforts are crucial for protecting ecosystems and human health.
Conclusion
Elements are the foundation of all matter and life itself. From the four major elements that dominate living matter to trace elements that quietly support essential biological functions, each plays a unique role. The periodic table provides a powerful framework for understanding these elements, their properties, and their interactions.
At the same time, awareness of harmful elements like mercury underscores the importance of responsible environmental management and ongoing scientific research. By studying elements and their behavior, we gain insight not only into the nature of matter but also into the delicate balance that sustains life on Earth.