Erythropoiesis is a precisely controlled process of development of mature, enucleated erythrocyte starting from multipotent precursor stem cell occurring in bone marrow.
The bone marrow produces around 1010 Red blood cells per hour during steady state hematopoiesis to keep the hemoglobin level within very strict bounds. However, when there is persistent bleeding or hemolysis, production might rise quickly. It is a multi-step process in which, stem cells first produce multipotent progenitor cells, then oligo-, then unilineage erythroid progenitors, and eventually morphologically distinct erythroid precursors and adult/mature red blood cells.
Erythropoiesis originates in the yolk sac, spleen, and liver during the early stages of fetal development. All erythropoiesis after birth takes place in the bone marrow. Erythropoiesis can take place in the bone marrow of the majority of children’s bones. Nevertheless, it only manifests in the bone marrow of the vertebrae, ribs, sternum, sacrum, pelvis, and proximal femur in adults.
Extramedullary hematopoiesis, i.e., hematopoiesis that occurs outside the bone marrow, can be triggered when erythropoiesis is insufficient in the marrow. This is frequently observed in haemoglobinopathies such thalassemia and myelofibrosis.
Stages of Erythropoiesis
The erythropoiesis process in the human body produces 2 million red blood cells per second. From the multipotent hematopoietic stem cells (HSC) to the adult erythrocyte, human erythropoiesis is a complicated, multi-step process.
1st Phase
The first step of erythroid differentiation starts with the differentiation of HSCs into more committed erythroid progenitor cells. The common myeloid progenitors and megakaryocytic-erythroid progenitor differentiates into the burst forming unit- erythroid (BFU-E), BFU-E are solely committed to erythrocyte lineage. BFU-Es undergo further differentiation to become Colony forming unit- erythroid (CFU-Es), and then terminal differentiation takes place.
2nd Phase
The differentiation of the proerythroblast-like nucleated precursors into basophilic, polychromatophilic, and orthochromatic erythroblasts is the second stage of erythroid maturation. Hemoglobin gradually builds up throughout this phase, cell size gradually shrinks, and nuclear condensation eventually leads to enucleation.
3rd Phase / Final Phase
The maturation of the reticulocyte into erythrocytes is the last stage of erythroid formation. The erythrocyte undergoes considerable membrane modification at this stage, giving it its characteristic biconcave form. The erythrocyte will continue to circulate in the bloodstream until it is eliminated by macrophages in the reticuloendothelial system.
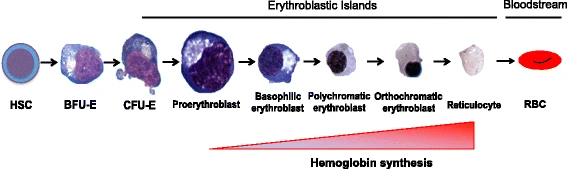
Source: https://molmed.biomedcentral.com/articles/10.1186/s10020-018-0011-z/figures/1
Regulation of Erythropoiesis
Growth factors, cytokines, and adhesion molecules between growing hematopoietic cells modulate the erythropoiesis process.
The earliest erythroid progenitors respond to SCF in particular, as well as TPO, GM-CSF, IL3, and IL11. KIT, a tyrosine kinase that transmits signals via PI-3 kinase, Src kinases, and PLC, is the receptor that SCF binds to.
At later stages, SCF collaborates with Erythropoietin (EPO) to promote the growth and development of the erythroid progenitors and may even contribute to the phosphorylation of the EPO receptor.
Erythroid cells in the latter phases of differentiation have lost their mitochondria, endoplasmic reticulum, and nucleus, which prevents them from proliferating.
Role of Erythropoietin in Erythropoiesis
EPO is a humoral cytokine that targets erythroid progenitor cells in the bone marrow. It is largely produced in the kidney and discharged into the bloodstream.
1% of the erythrocytes are eliminated daily and replaced by fresh cells in steady-state settings. Surprisingly, in response to hypoxic stress, which happens when an adequate oxygen supply to all tissues is hampered by a lack of functioning erythrocytes, the rate of erythropoiesis can dramatically rise from this baseline level. The principal defense against hypoxic stress is an increase in red cell synthesis.
EPO synthesis increases as a result, which influences the bone marrow to promote enhanced red blood cell formation. Hemoglobin levels rise as a result, which raises pO2 and thus causes EPO levels to decline. The feedback cycle has finished.
The erythropoietin receptor (EPO-R) becomes homodimerized when EPO binds to it. The EPO/EPO-R relationship is one of the main signaling routes that activates JAK2, which then phosphorylates and activates STAT5. It has been demonstrated that the JAK2/STAT5 pathway activates genes essential for the survival, proliferation, and differentiation of erythroid progenitors.
Erythropoietic Disorders
Direct impairment in medullary erythropoiesis, and even when the normal medullary erythropoiesis is present, altered Red Blood cells can be formed, which leads to different clinically relevant conditions. Some of the conditions due to erythropoietic disorders are described as follows.
β-thalassemia
An imbalance between the α- and β- globin chains occur in β -thalassemia as a result of a mutation in the β -globin gene. As a result, erythroid cells begin to accumulate unstable α-tetramers, which causes the developing red blood cell to undergo premature cell death. This results in poor differentiation of developing erythroblasts and inefficient erythropoiesis.
α-thalassemia
α -thalassemia is caused by an excess of β-chains that precipitate inside the growing red blood cell as a result of a decrease or lack of α-globin chains.
Sickle cell disease
An autosomal recessive condition known as sickle cell disease is brought on by a point mutation in the β -globin chain that results in the substitution of valine for glutamic acid at position 6. When periods of deoxygenation occur, the anomalous S hemoglobin leads to aberrant β-chain formation, which causes HbS molecules to polymerize together and drive the typical biconcave-shaped erythrocyte into an elongated, stiff form. Sickled erythrocytes result in aberrant endothelial interactions, persistent hemolysis, and vaso-occlusion in capillaries and arterioles.
Polycythemia cera
A clonal condition of myeloproliferation in the bone marrow is known as polycythemia vera (PV). It is characterized by increased red cell mass linked with the proliferation of the erythroid, megakaryocytic and granulocytic cell lines.
Underproduction of Red Blood Cells
Anemia is caused by a lack of red blood cell formation. Low hemoglobin concentration is the definition of anemia, yet males and women have different absolute levels.
In general, reduced red cell synthesis or increased red cell elimination can lead to anemia. The causes of reduced red cell production will be briefly discussed in this section.
Decreased red cell production might result from three major factors:
- absence of the “building blocks” necessary for manufacturing, such as an iron, folate, or B12 shortage.
- Failure of the stimulation, or chronic renal disease-related EPO insufficiency.
- failure of the bone marrow, including aplastic anemia.
Excess production of Red Blood Cells
A disorder called polycythaemia rubra vera can lead to an excessive production of red blood cells. The dysregulation at the level of the hemopoietic stem cell causes this myeloproliferative illness.
Red blood cell synthesis continues even when EPO manufacturing has been shut off. The JAK-2 mutation is present in over 95% of polycythaemia patients, and it often affects those over the age of 60. The majority of individuals with the condition live long and healthy lives, but constant monitoring is essential since it can raise the risk of thrombosis and because about 3% of cases progress to acute leukemia.
References
- https://teachmephysiology.com/immune-system/haematology/erythropoeisis/
- https://molmed.biomedcentral.com/articles/10.1186/s10020-018-0011-z
- https://www.frontiersin.org/articles/10.3389/fphys.2017.01076/full
- https://academic.oup.com/book/41095/chapter-abstract/351126239?redirectedFrom=fulltext
- http://perspectivesinmedicine.cshlp.org/content/3/4/a011601.full