Mary Elizabeth Brunkow (born 1961) is an American molecular biologist and program manager whose pioneering research uncovered the genetic basis of immune regulation. Best known for her co-discovery of the FOXP3 gene, a central regulator of regulatory T (Treg) cell development, Brunkow’s work laid the molecular foundation for understanding immune tolerance — the body’s ability to distinguish self from non-self. In recognition of this transformative contribution, she shared the 2025 Nobel Prize in Physiology or Medicine with Fred Ramsdell and Shimon Sakaguchi “for their discoveries concerning peripheral immune tolerance.”

Early Life and Education
Born and raised in the Pacific Northwest, Brunkow developed a deep curiosity about biology from an early age. She attended the University of Washington, where she earned her Bachelor of Science in Cell and Molecular Biology (1979–1983). The rigorous academic and research environment at UW introduced her to molecular genetics and laboratory science, sparking her lifelong interest in gene regulation and immunology.
Brunkow continued her academic journey at Princeton University, earning her Ph.D. in Molecular Biology (1984–1990). At Princeton, she was trained in cutting-edge techniques of molecular genetics, working on the regulation of gene expression in mammalian systems. This experience equipped her with a combination of scientific depth and technical precision that would become the hallmark of her later discoveries.
Early Career and the FOXP3 Discovery
After completing her doctorate, Brunkow joined Celltech R&D, Inc. in 1994 as a Senior Scientist and later Director of Genomics, where she spent a decade (1994–2004) conducting innovative research in molecular immunology and functional genomics. At Celltech, she was involved in studies that examined immune dysfunction in murine models — particularly a strain of mice known as scurfy mutants, which developed a severe autoimmune syndrome.
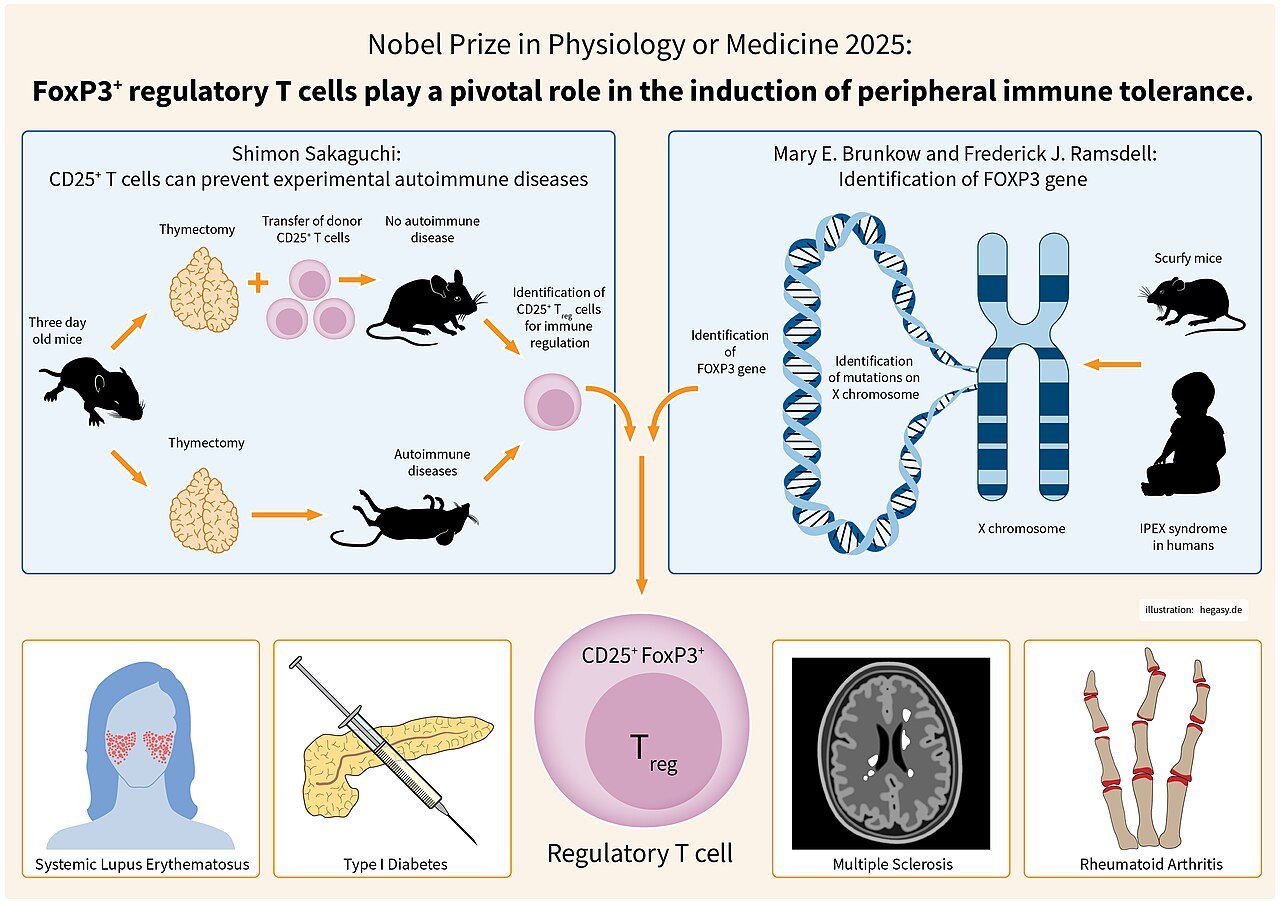
Brunkow and her colleagues undertook positional cloning of the gene responsible for the scurfy phenotype and discovered FOXP3, a member of the forkhead/winged-helix family of transcription factors. Her 2001 publication demonstrated that mutations in FOXP3 caused the loss of functional regulatory T cells, leading to uncontrolled immune activation. Soon after, parallel findings in humans revealed that mutations in FOXP3 result in IPEX syndrome (Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked) — a fatal autoimmune disorder.
This pivotal discovery provided molecular proof for the existence of a genetically defined subset of T cells responsible for immune tolerance, transforming immunology. The identification of FOXP3 and Tregs became a cornerstone of modern immunotherapy, influencing treatments for autoimmune diseases, organ transplantation, and cancer.
Translating Science into Application
Following her tenure at Celltech, Brunkow transitioned into biotech and translational research roles that bridged basic science with therapeutic development. In 2004, she served briefly as a Senior Scientist (contract) at Blue Heron Biotechnology, applying her molecular expertise to DNA synthesis and genomic analysis.
In 2006, she joined the Institute for Systems Biology (ISB) as a Science Writer, reflecting her ability to communicate complex scientific ideas across disciplines. She soon returned to research management, taking on increasingly senior roles in the biotechnology sector.
From 2008 to 2010, she served as Associate Director of Program Management at Trubion Pharmaceuticals, a Seattle-based biopharmaceutical company developing targeted biologics for autoimmune and inflammatory diseases. At Trubion, she managed interdisciplinary research programs focused on antibody engineering and immune modulation, continuing her career-long engagement with translational immunology.
Leadership at the Institute for Systems Biology
Since June 2009, Dr. Brunkow has held the position of Program Manager for Genetics at the Institute for Systems Biology (ISB) in Seattle, Washington. Over more than 16 years, she has played a central role in coordinating research programs that integrate genomics, systems biology, and computational modeling. ISB’s mission — to understand biology as an integrated and dynamic system — aligns closely with Brunkow’s vision of connecting molecular mechanisms to physiological outcomes.
Her leadership at ISB has extended into project design, data integration, and scientific communication. She has guided cross-functional teams of researchers, bioinformaticians, and clinicians to tackle complex biological questions, from immune response profiling to precision medicine approaches.
Key Project: Immune Repertoire Sequencing for Sepsis Detection
Among Brunkow’s notable ISB initiatives is her leadership in the “Pre-symptomatic Diagnosis of Sepsis via High-Throughput Immune Repertoire Sequencing (Rep-Seq)” project (initiated in 2013). The project explores how immune repertoire diversity — the variety of T and B cell receptors — changes in response to infection or stress.
Sepsis remains a leading cause of mortality in hospitalized patients, largely due to delayed diagnosis. Brunkow’s project hypothesizes that early changes in immune repertoire diversity can serve as a sensitive biomarker for sepsis, even before clinical symptoms appear. Using high-throughput sequencing of CDR3 regions from T-cell and B-cell receptors, her team measures shifts in immune diversity across serial patient samples. The approach represents an innovative systems-level application of immunogenomics — merging her foundational expertise in immune tolerance with real-world diagnostic innovation.
This research reflects Brunkow’s ongoing interest in host-pathogen interactions, immune system dynamics, and biomarker discovery — all central to modern precision medicine.
Professional Skills and Impact
Dr. Brunkow’s professional profile combines deep scientific knowledge with strong organizational and collaborative skills. Her expertise spans:
Genetics and Genomics
Immunology and Molecular Biology
Biotechnology Development
Program and Project Management
Scientific Communication and Writing
She has been endorsed by numerous colleagues for her technical and leadership strengths in Genetics and Biotechnology, with multiple endorsements from peers at the Institute for Systems Biology. Her scientific contributions, coupled with her ability to manage large interdisciplinary teams, have made her a respected figure in both academic and biotech communities.
Recognition and the Nobel Prize
In 2025, the Nobel Assembly recognized Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi with the Nobel Prize in Physiology or Medicine for their work elucidating the mechanisms of peripheral immune tolerance. The Nobel Committee cited Brunkow’s discovery of FOXP3 as a “genetic key to understanding the immune system’s self-regulatory capacity.” Her findings helped explain how the immune system prevents self-destruction and provided a framework for developing therapies that harness or modulate Tregs.
The recognition brought renewed attention to her career trajectory — from fundamental molecular biology to translational immunology and systems-level biomedical research. Universities and scientific institutions praised her collaborative spirit and her emphasis on integrating diverse disciplines to solve biological problems.
Personal Life and Legacy
Based in Seattle, Washington, Dr. Brunkow remains active in mentoring and supporting young scientists, particularly in genetics and immunology. Colleagues describe her as modest, precise, and deeply committed to scientific rigor.
Her legacy is defined not only by the FOXP3 discovery, which redefined immunology, but also by her sustained efforts to apply systems-level thinking to medicine. From revealing the genetic foundations of immune tolerance to developing genomic diagnostics for sepsis, Dr. Brunkow’s work exemplifies the integration of discovery science and translational impact.