The contraction of skeletal muscles is fundamental to all types of movements in animals, ranging from simple locomotion to complex manipulations. This contraction is driven by contractile proteins within muscle cells, particularly actin and myosin, which are organized into myofibrils. Myofibrils are further divided into sarcomeres, the functional units of muscle contraction. While actin forms the thin filaments, myosin constitutes the thick filaments, playing a pivotal role in the sliding filament model of muscle contraction. Myosin is not restricted to muscle tissue; it also contributes to various cellular functions in non-muscle cells, such as cell adhesion and migration.
In this article, we will explore the structure, synthesis, classification, and diverse roles of myosin, focusing on its crucial functions in muscle contraction and intracellular processes.
Structure of Myosin
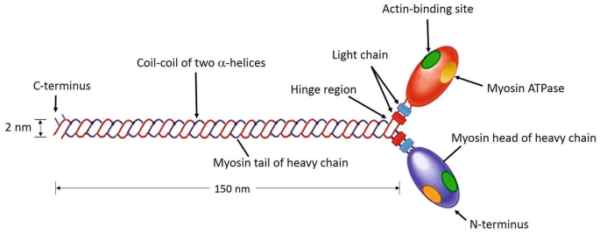
Myosin is a filamentous protein classified as a motor protein due to its ability to convert chemical energy from ATP hydrolysis into mechanical work. A single myosin molecule comprises six subunits: two heavy chains and four light chains. The structural organization of these chains is essential for its function.
- Heavy Chains: The two heavy chains are coiled around each other to form a double helix, constituting the tail of the myosin molecule. This tail forms the bulk of the myosin structure.
- Myosin Head: At one end of the heavy chains, they diverge and form globular structures known as myosin heads or cross-bridges. Each head contains an ATPase site and actin-binding sites.
- Light Chains: The globular heads are associated with two light chains each, which stabilize the structure of the heads.
Overall, the myosin molecule consists of two heads and one tail, forming a distinctive structure necessary for its function.
Domains of Myosin
To understand myosin’s function, it is helpful to consider its three primary domains:
- Head Domain: This domain is globular and consists of the end of the heavy chain and two light chains. It is responsible for binding to actin filaments and has ATPase activity crucial for muscle contraction.
- Neck Domain: Serving as a linker between the head and tail, the neck domain is essential for transducing the force generated by the heads to the tail. It also binds the light chains.
- Tail Domain: Formed by the coiled-coil structure of the heavy chains, the tail domain connects myosin molecules within a filament and interacts with cargo molecules in non-muscle cells.
Synthesis of Myosin
Myosin synthesis is a complex process involving multiple steps of gene expression.
Transcription
The process begins with transcription, where the DNA sequence of a myosin gene is copied into messenger RNA (mRNA). This occurs in the nucleus of muscle and non-muscle cells. Each gene corresponds to a specific myosin isoform, and only one gene is transcribed at a time.
Post-Transcriptional Modifications
To prepare the mRNA for translation, several modifications occur:
- 5′ Cap: A guanosine triphosphate (GTP) cap is added to the 5′ end of the mRNA to protect it from degradation and assist in translation initiation.
- Poly-A Tail: A polyadenylated tail is added to the 3′ end, further protecting the mRNA and aiding in its export to the cytoplasm.
Translation
Once in the cytoplasm, the mRNA is translated into a myosin protein. Ribosomes assemble around the mRNA, and transfer RNA (tRNA) molecules bring amino acids to the ribosome according to the mRNA sequence. This process continues until a stop codon is reached, signaling the end of translation. The newly synthesized myosin protein then undergoes post-translational modifications in the endoplasmic reticulum.
Post-Translational Modifications
Post-translational modifications are critical for the functional maturation of myosin:
- Phosphorylation: The addition of phosphate groups to serine, threonine, or tyrosine residues, catalyzed by myosin light chain kinases, can activate or deactivate myosin function.
- Nitration and Nitrosylation: The addition of nitrate or nitro groups can occur under pathological conditions, affecting myosin function and potentially leading to contractile dysfunction.
Classes of Myosin
Myosin is classified into several types based on its structure, location, and function. Key classes include:
- Myosin I: A monomeric protein involved in intracellular transport and membrane interactions.
- Myosin II: The classical muscle myosin responsible for muscle contraction, present in skeletal, smooth, and cardiac muscles.
- Myosin III: Found in Drosophila eyes, involved in light-dependent transduction.
- Myosin V: A dimeric protein that “walks” along actin filaments, crucial for intracellular transport.
- Myosin VI: Responsible for transporting endocytic vesicles within cells.
- Myosin VII: Involved in phagocytosis and spermatogenesis, and found in some sensory structures.
- Myosin VIII: Present in plant cells, regulating cell division and cytoplasmic flow.
- Myosin XI: A dimeric protein involved in organelle movement within plant cells.
Role in Muscle Contraction
Myosin plays a central role in muscle contraction through interactions with actin filaments. The mechanism varies slightly among skeletal, smooth, and cardiac muscles.
Skeletal Muscle
In skeletal muscle, myosin filaments are positioned in the center of sarcomeres, with actin filaments extending from either end. Myosin heads bind to actin filaments when binding sites are exposed due to calcium ion release. ATP hydrolysis powers the conformational change in myosin heads, resulting in a power stroke that pulls actin filaments towards the center of the sarcomere. This sliding filament mechanism causes muscle contraction.
Smooth Muscle
Smooth muscle contraction is regulated differently. Myosin filaments are interspersed with actin filaments attached to dense bodies. Unlike skeletal muscle, smooth muscle lacks troponin and tropomyosin. Instead, contraction is regulated by the phosphorylation of myosin light chains by myosin light chain kinase, activated by calcium ions. This process allows myosin heads to bind to actin and facilitate contraction.
Cardiac Muscle
Cardiac muscle contraction follows a similar mechanism to skeletal muscle, with myosin filaments arranged in sarcomeres. The sliding filament model applies here as well, with calcium ions triggering the contraction process.
Summary
Myosin is a critical protein in muscle contraction and various cellular processes. Its structure, comprising two heavy chains and four light chains, is essential for its function. The synthesis of myosin involves transcription, translation, and post-translational modifications. Myosin is classified into different types based on its function and location, including muscle and non-muscle forms.
In muscle contraction, myosin interacts with actin filaments through a sliding filament mechanism. While the fundamental process is similar across muscle types, the regulatory mechanisms differ between skeletal, smooth, and cardiac muscles. Understanding myosin’s structure and function provides insights into its diverse roles in both muscle physiology and cellular processes.
Actin: Structure, Function, and Dynamics – The Science Notes