Proteins are the molecular machines of life. Every cell in every living organism depends on proteins to survive, grow, communicate, and respond to the environment. From building tissues to catalyzing life-sustaining chemical reactions, proteins are essential biological macromolecules.
In this detailed, student-friendly guide, we will explore proteins, amino acids, peptide bonds, side chains (R groups), polypeptides, pH effects, and protein folding in depth—making it ideal for high school, undergraduate, and early medical science learners.
What Are Proteins?
A protein is a long chain of amino acids joined together by covalent peptide bonds and folded into a highly specific three-dimensional (3D) structure. This 3D structure is not random—it is precisely organized, and it determines the protein’s biological function.
Proteins are built from repeating units called amino acid residues. When amino acids link together, they form a chain known as a polypeptide backbone. The sequence of amino acids within this backbone contains all the information needed for proper folding.
Depending on their length and structural complexity, amino acid chains are classified as:
Oligopeptides (Peptides): Fewer than 20 amino acids
Polypeptides: Longer amino acid chains
Proteins: One or more folded polypeptides that carry out specific biological functions
Not all polypeptides are functional proteins. A protein must adopt a stable 3D conformation to perform its role effectively.
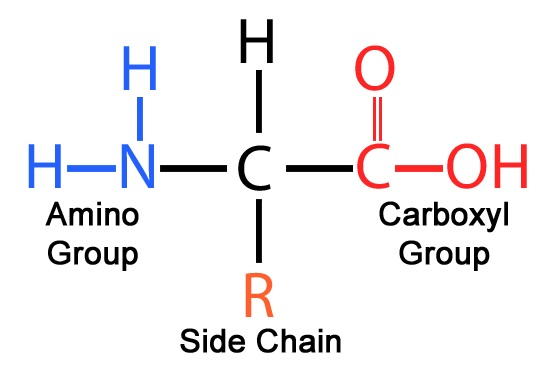
Amino Acids: The Fundamental Building Blocks
An amino acid is an organic molecule with a unique structural design. Each amino acid contains:
A central alpha (α) carbon
A carboxyl group (–COOH)
An amino group (–NH₂)
A hydrogen atom
A variable side chain (R group)
The R group is what makes each amino acid chemically distinct.
The Importance of the R Group
The side chain (R group) determines:
Whether the amino acid is hydrophobic or hydrophilic
Whether it carries a positive, negative, or neutral charge
Its ability to form hydrogen bonds
Its size and structural flexibility
For example:
Glycine has the simplest side chain—a single hydrogen atom.
Proline has a rigid ring structure that affects protein folding.
Tryptophan has a large aromatic side chain.
How Many Amino Acids Are Used in Proteins?
Although hundreds of amino acids exist in nature, only 21 amino acids are used to build proteins in eukaryotes (with 20 directly encoded by the genetic code).
They are represented using:
Three-letter abbreviations: Gly, Val, Pro
One-letter abbreviations: G, V, P
The specific order of amino acids—known as the primary structure—determines everything about the final protein.
Peptide Bonds and Dehydration Synthesis
How Do Amino Acids Form Proteins?
Amino acids link together through a chemical reaction called dehydration synthesis (also known as a condensation reaction).
During this process:
The amino group (–NH₂) of one amino acid reacts with
The carboxyl group (–COOH) of another amino acid
A molecule of water (H₂O) is released
A strong covalent peptide bond is formed
This peptide bond is stable and forms the backbone of proteins.
Directionality: N-Terminus and C-Terminus
Polypeptides have directionality:
N-terminus: The beginning, with a free amino group (NH₃⁺)
C-terminus: The end, with a free carboxyl group (COO⁻)
Protein sequences are always written from N-terminus to C-terminus, reflecting how they are synthesized inside cells.
Classification of Amino Acids by Side Chains
The chemical properties of side chains strongly influence protein folding and function. Amino acids are commonly grouped into four major categories:
1. Negative Polar (Acidic) Amino Acids
Contain a carboxyl group in the side chain
Carry a negative charge at physiological pH
Example: Aspartic acid, Glutamic acid
Participate in ionic interactions
2. Positive Polar (Basic) Amino Acids
Contain amino groups in the side chain
Carry a positive charge at neutral pH
Often interact with negatively charged molecules like DNA
3. Polar Uncharged Amino Acids
Hydrophilic
Form hydrogen bonds
Often located on protein surfaces
4. Nonpolar (Hydrophobic) Amino Acids
Lack charged or strongly polar groups
Repel water
Typically buried inside the protein core
Range from simple (glycine) to bulky (tryptophan)
Hydrophobic vs Hydrophilic Distribution
In aqueous environments:
Hydrophobic amino acids cluster in the interior
Hydrophilic amino acids face outward
This organization stabilizes protein structure and is a major driving force behind folding.
The Effect of pH on Amino Acid Chemistry
Amino acids are amphoteric, meaning they can act as both acids and bases.
Their behavior depends on environmental pH.
At Low pH (Acidic Conditions, ~pH 2)
Amino group: –NH₃⁺
Carboxyl group: –COOH
Molecule carries an overall positive charge
At High pH (Alkaline Conditions, ~pH 13)
Amino group: –NH₂
Carboxyl group: –COO⁻
Molecule carries an overall negative charge
At Physiological pH (~7.4)
Amino group: –NH₃⁺
Carboxyl group: –COO⁻
Forms a zwitterion (both positive and negative charges)
This dual charge allows amino acids to:
Form hydrogen bonds
Participate in ionic interactions
Stabilize complex protein structures
pH changes can disrupt protein structure—a process called denaturation.
Polypeptide vs Protein: Understanding the Difference
Although often used interchangeably, there is a distinction:
A polypeptide is a linear chain of amino acids.
A protein is a folded polypeptide (or multiple polypeptides) capable of performing a biological function.
Protein folding produces higher levels of structure:
Primary Structure: Amino acid sequence
Secondary Structure: Alpha-helices and beta-sheets
Tertiary Structure: 3D folding of a single chain
Quaternary Structure: Multiple polypeptide subunits
Only when properly folded does a polypeptide become a functional protein.
Protein Size and Diversity
Proteins vary tremendously in length and complexity.
For example:
Thyroid-releasing hormone contains 234 amino acids.
Connectin, a massive elastic muscle protein, contains over 34,000 amino acids.
This range illustrates the extraordinary diversity of protein architecture.
Each protein is unique because:
The number of amino acids differs
The sequence of amino acids differs
The side chain interactions differ
The final 3D folding pattern differs
Even a single amino acid change can dramatically alter function.
Why Proteins Are Essential for Life
Proteins are one of the four fundamental biological macromolecules, along with:
Carbohydrates
Nucleic acids
Lipids
They perform critical roles in nearly every biological process:
Structural Support
Collagen strengthens connective tissue
Movement
Actin and myosin drive muscle contraction
Catalysis
Enzymes accelerate biochemical reactions
Transport
Membrane proteins move molecules across cell membranes
Immune Defense
Antibodies recognize and neutralize pathogens
Without proteins, cellular life would be impossible.
Key Learning Points for Students
Proteins are composed of amino acids linked by peptide bonds.
The R group determines amino acid chemical behavior.
Peptide bonds form through dehydration synthesis.
The N-terminus and C-terminus define protein directionality.
pH influences amino acid charge and protein stability.
Hydrophobic interactions drive protein folding.
Structure determines function.