Plasmid DNA is a versatile tool in molecular biology, often used for various genetic manipulations and studies. Restriction enzyme digestion of plasmid DNA is a crucial technique that allows researchers to precisely cleave the DNA at specific sites, facilitating downstream applications such as cloning, sequencing, and mapping. In this article, we will delve into the principles, procedures, reagents, and tips associated with restriction enzyme digestion of plasmid DNA.
Principle of Restriction Enzyme Digestion
Restriction digestion, also known as restriction endonuclease digestion, involves the cleavage of DNA at specific recognition sites by restriction enzymes. These enzymes recognize short, palindromic sequences of nucleotides and catalyze the hydrolysis of phosphodiester bonds in the DNA backbone. This results in the formation of fragments with either blunt ends or sticky ends, depending on the enzyme and its cutting mechanism.
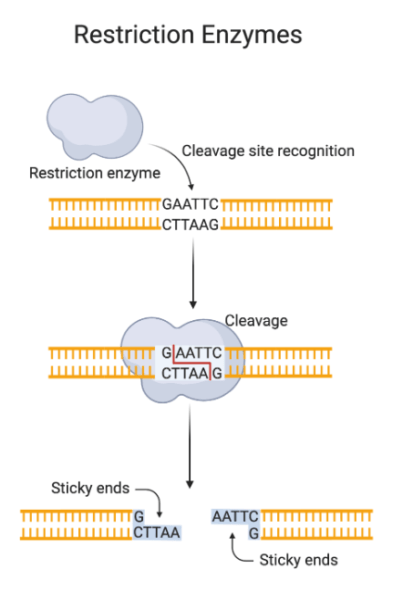
Importance and Applications
Restriction digestion of plasmid DNA is essential for various molecular biology techniques, including:
- Molecular cloning: Restriction digestion allows for the preparation of DNA fragments that can be subsequently ligated into vectors for cloning purposes.
- Sequence analysis: It provides a method for indirectly obtaining sequence information by analyzing the fragment patterns generated after digestion.
- Diagnostic digests: Used to quickly check the identity of a plasmid by verifying the presence or absence of specific restriction sites.
Reagents Required
To perform restriction enzyme digestion of plasmid DNA, the following reagents are typically used:
- Plasmid DNA: The DNA template to be cleaved.
- Restriction enzyme(s): Enzymes that recognize and cleave specific DNA sequences.
- Restriction buffer: Provides optimal pH and ionic conditions for enzyme activity.
- BSA (Bovine Serum Albumin): Optional, used to stabilize certain restriction enzymes.
- Gel loading dye and electrophoresis buffer: For analyzing the digested DNA fragments by gel electrophoresis.
Procedure of Restriction Enzyme Digestion of Plasmid DNA
1. Selection of Restriction Enzymes:
Choose the appropriate restriction enzyme(s) based on the desired cleavage sites and the nature of the DNA fragment required.
Pro-Tip: Utilize sequence analysis tools to predict enzyme cutting sites accurately.
2. Preparation of Reaction Mix:
Combine the plasmid DNA, restriction enzyme(s), buffer, and any additional components such as BSA in a microcentrifuge tube.
Pro-Tip: Determine the appropriate reaction buffer and enzyme concentration based on manufacturer instructions.
3. Incubation:
Incubate the reaction mix at the optimal temperature for enzyme activity, typically 37°C, for a specific duration.
Pro-Tip: Ensure proper incubation time for complete digestion, considering factors such as DNA concentration and enzyme activity.
4. Analysis:
After incubation, analyze the digested DNA fragments using gel electrophoresis to visualize the cleavage pattern and fragment sizes.
Tips for Success in Restriction Enzyme Digestion of Plasmid DNA
- Proper enzyme handling: Ensure enzymes are stored and handled according to manufacturer’s instructions to maintain activity.
- Optimization of reaction conditions: Adjust buffer conditions, enzyme concentration, and incubation time for optimal digestion efficiency.
- Consideration of methylation sensitivity: Choose enzymes that are compatible with the methylation status of the DNA.
- Quality control: Include positive and negative controls in each experiment to validate digestion efficiency.
- Documentation and analysis: Document experimental details and analyze gel images accurately to interpret results effectively.
Conclusion
Restriction enzyme digestion of plasmid DNA is a fundamental technique in molecular biology, enabling precise manipulation and analysis of genetic material. By understanding the principles, optimizing experimental conditions, and following best practices, researchers can successfully employ this technique for a wide range of applications, advancing our understanding of genetics and biotechnology. In summary, restriction digestion of plasmid DNA serves as a cornerstone in molecular biology research, empowering scientists to explore and manipulate the intricacies of the genetic code. Through careful experimentation and innovation, this technique continues to drive advancements in biotechnology and beyond.
Troubleshooting Plasmid DNA Digestion
Plasmid DNA digestion, a critical step in molecular biology experiments, can sometimes encounter challenges leading to incomplete or unsuccessful cleavage. Understanding potential issues and implementing effective troubleshooting strategies is essential for obtaining reliable results. In this guide, we’ll explore common problems encountered during plasmid DNA digestion and provide practical solutions to address them.
Problem: Incomplete Digestion
Possible Causes:
- Suboptimal Incubation Conditions: Incorrect temperature or duration of incubation.
- Enzyme Inactivation: Enzyme denaturation due to improper handling or storage.
- Suboptimal Buffer Conditions: Incorrect buffer pH, salt concentration, or presence of inhibitors.
Solutions:
- Optimize Incubation Conditions: Ensure the reaction is maintained at the optimal temperature for the specified duration according to the manufacturer’s instructions.
- Handle Enzymes Carefully: Store enzymes properly at recommended temperatures and avoid excessive freeze-thaw cycles. Use an ice bucket immediately after removal from the freezer to prevent denaturation.
- Verify Buffer Compatibility: Ensure the buffer used is suitable for the chosen enzyme(s) and adjust pH or salt concentration if necessary.
Problem: Star Activity
Possible Causes:
- High Glycerol Concentration: Presence of glycerol in the reaction buffer may induce non-specific cleavage.
- Suboptimal Reaction Conditions: Enzyme activity influenced by temperature, pH, or ionic strength variations.
Solutions:
- Optimize Reaction Conditions: Maintain consistent reaction conditions, including temperature and buffer composition, to minimize non-specific cleavage.
- Use Fresh Buffers: Prepare fresh reaction buffers without excess glycerol to prevent unwanted enzymatic activity.
Problem: Methylation Sensitivity
Possible Causes:
- Methylation of DNA: Presence of methyl groups on specific DNA bases may inhibit enzyme cleavage.
- Methylation of Enzymes: Some restriction enzymes may be sensitive to methylation, affecting their activity.
Solutions:
- Use Methylation-Sensitive Enzymes: Select enzymes that are compatible with the methylation status of the DNA sample.
- Prevent Methylation: Perform DNA isolation from bacterial strains lacking methylase activity or use specific methylation-sensitive enzymes.
Problem: Contaminants or Inhibitors
Possible Causes:
- Contaminants in DNA Sample: Presence of contaminants such as phenol, chloroform, or salts may interfere with enzyme activity.
- Presence of Detergents: Detergents from DNA isolation kits may inhibit enzyme activity.
Solutions:
- Purify DNA Sample: Use purification methods to remove contaminants before digestion.
- Avoid Detergents: If using commercial DNA isolation kits, ensure thorough washing to remove residual detergents.
Problem: Poor Ligation Efficiency
Possible Causes:
- Blunt-Ended Fragments: Blunt-ended DNA fragments may have lower ligation efficiency compared to fragments with cohesive ends.
- Mismatched Sticky Ends: Incompatible sticky ends may result in inefficient ligation.
Solutions:
- Consider Overhang Compatibility: Choose enzymes generating compatible sticky ends for efficient ligation.
- Use T4 DNA Ligase: Use T4 DNA ligase, which can ligate both blunt and cohesive ends, to improve ligation efficiency.
Conclusion
Troubleshooting plasmid DNA digestion requires a systematic approach to identify and address potential issues. By understanding the underlying causes of incomplete digestion or inefficient ligation, researchers can implement appropriate solutions to optimize experimental outcomes. Through careful optimization of reaction conditions, enzyme selection, and sample preparation techniques, reliable digestion of plasmid DNA can be achieved, facilitating downstream molecular biology applications with confidence.
References
- New England Biolabs (NEB). (n.d.). Restriction Enzyme Digestions: Protocol. Retrieved from NEB Website
- Addgene. (n.d.). Sequence Analyzer. Retrieved from Addgene Website
- Roberts RJ. (2005). How restriction enzymes became the workhorses of molecular biology. Proceedings of the National Academy of Sciences of the United States of America, 102(17), 5905–5908. https://doi.org/10.1073/pnas.0500923102
- Wilson GG, Murray NE. (1991). Restriction and modification systems. Annual Review of Genetics, 25, 585–627.
- Pingoud A, Jeltsch A. (2001). Structure and function of type II restriction endonucleases. Nucleic Acids Research, 29(18), 3705–3727.
- Sambrook J, Russell DW. (2001). Molecular cloning: A laboratory manual (3rd ed.). Cold Spring Harbor Laboratory Press.
Dear Madam / Sir,
Warm greetings from the National Digital Library of India (NDLI). Please visit NDLI at https://www.ndl.gov.in/ or https://ndl.iitkgp.ac.in/.
NDLI is a digital library of the Ministry of Education, Government of India, developed by the Indian Institute of Technology Kharagpur, which is focussed on the education domain though it hosts contents of other domains, such as career, cultural heritage, as well. NDLI is an aggregator which integrates primarily open-access and free contents from other publicly hosted websites at the metadata level. This means NDLI only stores indexed metadata of contents and doesn’t store the actual content in its repository. Content view/download by NDLI users happens from the website of the respective content sources in the NDLI Frame as shown below in an example screenshot.
Thus access by NDLI users increase usage/site hit of the website of the content source. By granting permission for the NDLI to host your metadata, you would make a profound impact on the lives of millions of individuals who seek access to quality educational materials.
NDLI is available as a website and Mobile App (Android and iOS). It currently hosts more than 95 million content and daily content view/download from the NDLI site is more than 400,000. NDLI is open to all and free and anybody can view/download content from NDLI without even registering or logging in, though registration/login is recommended for a better user experience. NDLI currently has more than 8 million registered users.
Details about the project are available at: http://project.ndl.gov.in/.
Regular updates on the project are available at: https://www.facebook.com/NDLIndia/.
NDLI has explored your source “Online Biology Notes” ( https://www.onlinebiologynotes.com/) and proposes to integrate it into NDLI at the metadata level. While the integration will enrich the repository of the NDLI, it will also increase the usage/site hit of the “ Online Biology Notes” .
For this purpose, NDLI proposes to acquire metadata of “ Online Biology Notes ” by crawling your website. Otherwise, you may propose to make the metadata available to NDLI as XML, JSON, and CSV files.
Thank you for being part of our journey.
Please let us know your views on the above and advise.
Thanks & Regards
Dolan Santra
Project Engineer
NDLI, IIT KGP
Mob: +91 9932533765