The human immune system is a marvel of biological engineering, equipped with specialized cells that identify and eliminate pathogens with impressive precision. One key player in this system is the B cell—a type of white blood cell responsible for producing antibodies. But how exactly do B cells develop and mature to become the guardians of our adaptive immunity? Let’s explore the complex and fascinating journey of B cell development and maturation, from their origins in the bone marrow to their roles in defending the body against infections.
The Origins of B Cells: From Stem Cells to Lymphoid Commitment
B cell development begins during fetal life and continues throughout adulthood. It starts when multipotent progenitor cells (MPPs), which can give rise to many cell types, migrate first to the fetal liver and then to the bone marrow, where hematopoiesis occurs.
Within the bone marrow microenvironment, MPPs differentiate into common lymphoid progenitors (CLPs). These CLPs further specialize into LCA-2 cells (common lymphoid 2 progenitors), which are committed to the B cell lineage. This commitment is guided by signals from stromal bone marrow cells, including important cytokines such as interleukin-7 (IL-7) and Fms-like tyrosine kinase 3 ligand (Flt3-L).
This process is tightly controlled by a set of transcription factors including:
PU.1 and IKAROS: Early regulators of lymphoid lineage commitment.
E2A, EBF1 (early B cell factor 1), and PAX5: Critical for B cell identity.
IRF8: Involved in early B cell gene expression.
Together, these signals and transcription factors kick-start the process that transforms stem cells into fully functional B lymphocytes.
Crafting the B Cell Receptor (BCR): A Molecular Assembly Line
The defining feature of a B cell is its B cell receptor (BCR)—a membrane-bound version of the antibody it will eventually secrete. This receptor is what allows B cells to detect specific antigens. But before a B cell can respond to any pathogen, it must first construct a unique BCR.
The genetic blueprints for the BCR reside in immunoglobulin (Ig) gene segments. These segments—V (variable), D (diversity), J (joining), and C (constant)—undergo a process known as VDJ recombination. This genetic rearrangement, driven by enzymes RAG1/2 (recombinase activating genes) and TdT (terminal deoxynucleotidyl transferase), generates an enormous diversity of BCRs, enabling the immune system to recognize virtually any pathogen.
B cell development follows these sequential stages:
Early pro-B cell: The D and J segments of the heavy chain gene are joined.
Late pro-B cell: A V segment is added, completing the VDJ recombination.
Pre-B cell: The newly formed heavy chain is tested with a surrogate light chain (SLC), made of λ5 and VpreB, forming the pre-BCR.
The pre-BCR complex includes Ig-α and Ig-β signaling proteins. Successful signaling at this stage is critical—it shuts down further heavy chain recombination (a process called allelic exclusion) and triggers proliferation.
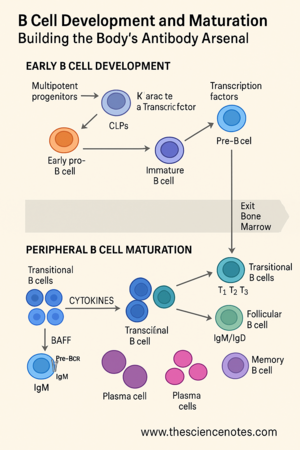
From Pre-B Cells to Immature B Cells
After successful heavy chain assembly, B cells enter the large pre-B cell stage, characterized by rapid proliferation. Once they stop dividing, they become small pre-B cells and re-express RAG1/2 to start light chain gene rearrangement at either the kappa or lambda loci.
A successful light chain pairing with the heavy chain forms a complete BCR, which is expressed as IgM on the cell surface, marking the immature B cell stage. This BCR is then tested against self-antigens in the bone marrow to eliminate potentially harmful self-reactive clones.
Although much of this knowledge comes from mouse models, human B cell development follows a similar pattern. However, a key difference is that IL-7 is critical in mice but not essential in humans (LeBien, 2000).
The Journey Continues: Peripheral B Cell Maturation
Once they pass self-reactivity checks, immature B cells exit the bone marrow and enter circulation. These are called transitional B cells, which represent the final maturation phase before becoming part of the functional immune system.
Transitional B cells are divided into three subsets:
T1 B cells
T2 B cells
T3 B cells
The spleen plays a central role here. T1 cells localize in the red pulp, while T2 cells populate splenic follicles. During this transition, selection ensures that only cells with low affinity for self-antigens survive.
Key Signals for Transitional B Cell Maturation
Maturation depends on signaling through the BAFF (B cell activating factor) receptor system, including:
BAFF-R (BR3)
TACI
APRIL
BCMA
These signals help determine B cell fate and promote survival and differentiation. Importantly, BAFF levels are tightly regulated—excess signaling can lead to autoimmunity, while too little causes immunodeficiency.
Choosing a B Cell Fate: Follicular, Marginal Zone, and Germinal Center B Cells
Mature B cells fall into several specialized subtypes, each with distinct roles:
Follicular (FO) B cells
These are the most common type. They circulate between the bone marrow and secondary lymphoid organs (like lymph nodes and spleen) and are involved in T cell-dependent antibody responses.Marginal Zone (MZ) B cells
Located in the spleen’s marginal zone, these cells are strategically positioned to respond to blood-borne pathogens, often without T cell help.Germinal Center (GC) B cells
Upon encountering antigens, follicular B cells enter germinal centers where they undergo:Clonal expansion
Somatic hypermutation
Affinity maturation
These processes fine-tune the antibody specificity and increase antigen affinity, ensuring an effective immune response.
Long-Term Immunity: Plasma Cells and Memory B Cells
After a successful germinal center reaction, B cells differentiate into two major effector types:
Plasma Cells:
These are antibody-secreting factories. Some plasma cells are short-lived and reside in secondary lymphoid organs, while others migrate to the bone marrow and persist for years, providing long-term immunity.Memory B Cells:
These cells circulate in the blood and lymphoid tissues, ready to respond more rapidly and effectively if the same antigen is encountered again.
Each B cell subset has distinct surface markers and transcription factors that guide their identity and function. For example:
PAX5, EBF, and Oct2 help maintain B cell identity.
BCL6 is essential in germinal center B cells.
BLIMP1, IRF4, and XBP1 drive plasma cell differentiation.
OBF1 and SPI-B are involved in memory B cell development.
Comparing Human and Mouse B Cell Maturation
Although similar in principle, there are species-specific differences between mouse and human B cell development. For instance:
Mice are more dependent on IL-7.
Marker profiles vary slightly (e.g., CD1d in mice vs. CD1c in humans for MZ B cells).
The locations and dynamics of transitional stages are more well-defined in mouse models.
Such comparisons help researchers fine-tune therapies and vaccines, as many preclinical models are mouse-based.
Conclusion
The journey from a multipotent stem cell to a specialized B cell capable of producing high-affinity antibodies is intricate, highly regulated, and essential for immune defense. Every checkpoint—from VDJ recombination to germinal center selection—ensures that B cells can recognize foreign threats while avoiding self-reactivity.
As we continue to uncover the finer details of B cell development and maturation, we gain valuable insights into treating autoimmune diseases, immunodeficiencies, and designing effective vaccines. In an era increasingly shaped by our understanding of immunology, B cells stand as powerful allies in protecting human health.