What is DNA?
DNA, short for deoxyribonucleic acid, serves as the genetic material and is composed of four nucleotides: dATP (deoxyadenosine triphosphate), dGTP (deoxyguanosine triphosphate), dCTP (deoxycytidine triphosphate), and TTP (thymidine triphosphate). We will learn about DNA base pairing and Chargaff’s rules in this article.
These nucleotides consist of three essential components:
- Nitrogenous bases (adenine, thymine, cytosine, and guanine), which are the four DNA bases.
- Sugar (deoxyribose), forming the sugar moiety.
- Phosphate groups.
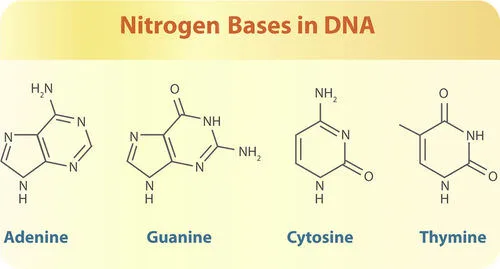
When the nitrogenous bases combine with the sugar moiety, they become nucleosides. Nucleotides are formed when phosphate groups are added to nucleosides.
Since DNA is double-stranded, the nucleotides on one strand pair with the nucleotides on the other strand through nitrogenous base pairing. This pairing creates strong hydrogen bonds that hold the two DNA strands together. These base-pairing interactions follow specific and universal rules, known as base-pairing rules.
While DNA and RNA are both nucleic acids, DNA contains deoxyribose sugar, whereas RNA contains ribose sugar. Consequently, the nucleotides in RNA are ATP, GTP, CTP, and UTP. Notably, RNA base pairs differ from DNA base pairs as adenine pairs with uracil instead of thymine.
What is Base pair?
A base pair is a fundamental unit in DNA, consisting of two complementary nucleotide bases that combine to create a “rung” in the DNA ladder structure. DNA is composed of two intertwined strands, resembling a twisted ladder known as a double helix. Each strand features a backbone comprising alternating sugar (deoxyribose) and phosphate groups. Connected to each sugar molecule is one of four bases: adenine (A), cytosine (C), guanine (G), or thymine (T).
The pairing of these bases is essential for DNA’s structure and function. The two strands are held together by hydrogen bonds that form between specific base pairs. Adenine pairs exclusively with thymine, forming two hydrogen bonds, while cytosine pairs exclusively with guanine, forming three hydrogen bonds. This complementary base pairing mechanism ensures the stability and fidelity of DNA replication and transmission of genetic information.
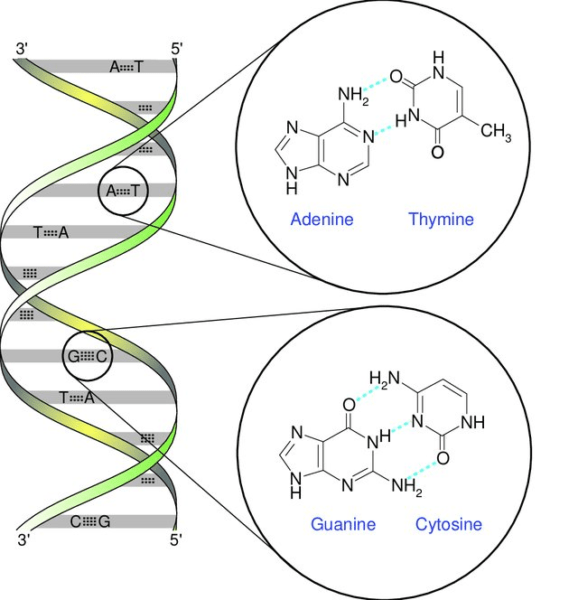
DNA Base pairing rules
Nucleotides, present on complementary strands of the DNA double helix, engage in chemical bonding with each other, resembling the rungs of a ladder. These chemical bonds play a crucial role in maintaining the integrity of the DNA structure by keeping the two strands held together. Within DNA, there exist four nucleotides, or bases: adenine (A), cytosine (C), guanine (G), and thymine (T). These bases selectively pair up, with adenine pairing specifically with thymine, and guanine pairing with cytosine.
Chargaff’s rules
Chargaff’s rules, also known as Chargaff’s ratios, are a set of observations made by the biochemist Erwin Chargaff in the 1940s that revolutionized our understanding of DNA’s structure and function. These rules played a crucial role in the discovery of the DNA double helix and paved the way for modern genetics and molecular biology.
Chargaff’s first rule
Chargaff’s first rule, often referred to as base pairing, states that in a double-stranded DNA molecule, the amount of adenine (A) is equal to the amount of thymine (T), and the amount of guanine (G) is equal to the amount of cytosine (C). This means that the total purine content (A + G) is equal to the total pyrimidine content (T + C). Chargaff’s careful analysis of DNA samples from various species consistently revealed these equalities, suggesting a fundamental relationship between the base pairs.
Chargaff’s second rule
The second rule states that while the proportions of adenine, thymine, guanine, and cytosine may vary between different species, they remain consistent within a particular species. For example, in humans, the base composition of DNA is approximately 30% adenine, 30% thymine, 20% guanine, and 20% cytosine. This rule emphasized the importance of understanding the unique characteristics of DNA for each species.
Significance of Chargaff’s rule
These rules provided crucial clues about the structure of DNA. Chargaff’s observations suggested that DNA is composed of two complementary strands, where adenine pairs with thymine through two hydrogen bonds, and guanine pairs with cytosine through three hydrogen bonds. This insight was instrumental in the development of James Watson and Francis Crick’s double helix model of DNA, which they proposed in 1953. Watson and Crick’s model elegantly explained how the base pairs fit together, forming the iconic twisted ladder-like structure of DNA.
Base pairing in DNA stability and function
The base pairing arrangement in DNA is crucial for its stability and function. The hydrogen bonds between complementary base pairs hold the two strands of DNA together, providing structural integrity. The specific pairing of A with T and G with C ensures that the width of the DNA double helix remains consistent, as both A-T and C-G pairs have a length of 10.85Å, fitting neatly between the sugar-phosphate backbones.
Role in DNA replication and transcription
These base pairings also play a significant role in DNA replication and transcription. They act as a protective mechanism, preventing other molecules from accessing the nitrogenous bases until the hydrogen bonds between the base pairs are broken. Specific enzymes can easily break these hydrogen bonds to facilitate essential cellular processes. For example, during DNA replication, the enzyme DNA helicase unwinds the double helix by separating the hydrogen-bonded base pairs, allowing each strand to serve as a template for the synthesis of a new complementary strand.
It’s worth noting that the G-C base pair has three hydrogen bonds, while the A-T base pair has two. This difference in hydrogen bonding contributes to the stability of DNA and affects its physical properties. DNA with a higher percentage of G-C pairs requires more energy to separate the DNA strands compared to DNA with a similar percentage of A-T pairs. This difference in stability can have implications for various biological processes and the overall functioning of DNA within an organism.
In summary, Chargaff’s rules were groundbreaking in unraveling the mysteries of DNA. His observations provided crucial insights into the base composition of DNA, revealing the equalities between A and T and between G and C. These findings, combined with subsequent research by Watson and Crick, led to the development of the double helix model of DNA. Chargaff’s rules laid the foundation for our understanding of DNA’s structure, function, and its vital role in genetics and molecular biology
Base Analogs in Medicine: Enhancing DNA Replication and Fighting Viral and Cancerous Diseases
Base analogs play a vital role in ensuring accurate DNA replication, as correct base pairing is crucial for this process. These specialized molecules have the ability to substitute the standard DNA bases during DNA replication. Moreover, base analogs have emerged as effective agents in the treatment of various viral infections and cancers, including hepatitis, herpes, and leukemia. Acyclovir, also known as Acycloguanosine, represents a prominent base analog derived from guanine. It is widely utilized in combating the herpes simplex virus.
Acyclovir Mechanism of Action
Acyclovir functions by mimicking guanine during DNA replication, thereby binding to adenine as expected. However, due to the absence of a 3′ end of the nucleotide, DNA polymerase is unable to proceed with forming subsequent base pairs. Consequently, replication comes to a halt, preventing the virus from replicating further.
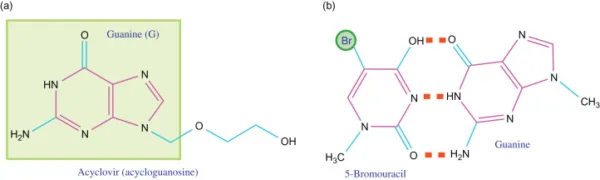
Base analogs offer a promising avenue for developing antiviral and anticancer therapies. By leveraging their ability to disrupt DNA replication, these compounds hold great potential in combating a range of diseases. Acyclovir serves as a prime example, showcasing the effectiveness of base analogs in the treatment of viral infections.
Incorporating base analogs into medicine marks a significant advancement in our ability to combat diseases at the molecular level. Understanding their mechanism of action and their potential applications brings us closer to developing targeted therapies for a multitude of conditions, improving patient outcomes, and advancing the field of medicine.
Learn more