Author: Binod G C
What are Carbapenems?
Carbapenems are a class of broad-spectrum antibiotics that are highly effective against a wide range of bacteria, including those that are resistant to other antibiotics. They are beta-lactam antibiotics, which means that they contain a beta-lactam ring in their molecular structure. This ring allows them to interfere with bacterial cell wall synthesis, ultimately leading to the death of the bacteria. Carbapenems are often used as a last resort treatment for serious bacterial infections when other antibiotics have failed, but their overuse can contribute to the development of antibiotic resistance, which is a growing concern in healthcare settings.
Timeline of Development
Here is a timeline of the development of carbapenems:
- Late 1960s: Bacterial beta-lactamases begin to emerge and threaten the efficacy of penicillin. The search for beta-lactamase inhibitors begins in earnest.
- 1976: The first beta-lactamase inhibitors, olivanic acids, are discovered. They are natural products produced by the Gram-positive bacterium Streptomyces clavuligerus and act as broad-spectrum beta-lactams. However, due to chemical instability and poor penetration into the bacterial cell, they are not further pursued.
- Shortly thereafter: Two superior beta-lactamase inhibitors are discovered. Clavulanic acid is the first clinically available beta-lactamase inhibitor, and thienamycin is the first carbapenem and would eventually serve as the parent or model compound for all carbapenems.
- 1980s: A series of other compounds are identified, including imipenem, which becomes the first carbapenem to be approved for clinical use.
- 1990s-2000s: More carbapenems are developed, including meropenem, ertapenem, and doripenem.
- Present day: Carbapenems are an important class of antibiotics used to treat serious bacterial infections, particularly those caused by multidrug-resistant bacteria. However, their overuse and misuse can contribute to the development of antibiotic resistance, which is a growing concern in healthcare settings.

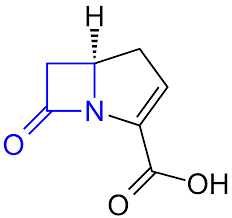
Structure of Carbapenems
- Carbapenems are beta-lactam antibiotics.
- They have a bicyclic nucleus with a five-membered ring that includes a beta-lactam ring.
- The carbapenem nucleus has a double bond between the C2 and C3 positions and contains a carbon atom at the C1 position.
- The C5 position is usually substituted with a thiazolidine ring, which gives carbapenems their characteristic stability to beta-lactamases.
- The C6 position is usually substituted with an ethoxy group.
- The R1 and R2 side chains, which can vary depending on the specific carbapenem, are attached to the C2 and C6 positions.
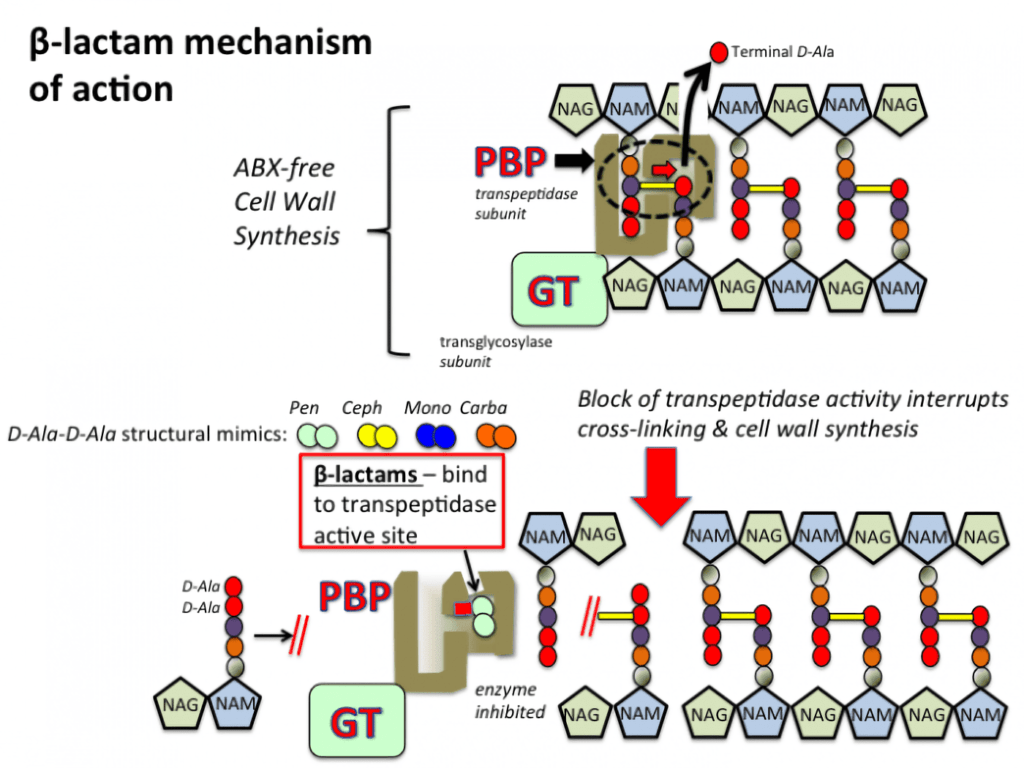
Mechanism of Action of Carbapenems
- Carbapenems are a group of beta-lactam antibiotics that irreversibly bind to PBPs in bacterial cells and inhibit cell wall synthesis.
- PBPs are responsible for cross-linking peptidoglycan chains in the bacterial cell wall.
- Binding of antibiotics to PBPs inhibits bacterial cell wall synthesis, causing cell lysis and death.
- Carbapenems have a strong affinity for PBPs, hindering cell wall synthesis and dissolving bacteria. They have a selective bactericidal effect on bacteria and have little toxicity to the host, as mammalian cells lack cell walls.
- These antibiotics are effective against a broad spectrum of bacteria, including those resistant to other beta-lactam antibiotics.
- Imipenem binds strongly to PBP, whereas meropenem targets PBP2 and PBP3. Panipenem targets PBP1 and PBP3 on Staphylococcus aureus and PBP2 on Escherichia coli, S. mucilaginosus, and Pseudomonas aeruginosa.
- Carbapenems are effective even at a broad range of pH levels (pH 5.5~8.5) and can quickly penetrate into bacterial cells.
- Misuse and overuse of antibiotics can contribute to antibiotic resistance, making it essential to use them judiciously, adhering to proper dosing regimens, and only when necessary.
- Carbapenems are a vital class of antibiotics with a unique mechanism of action that is effective against a wide range of bacterial infections, making them an essential tool in the fight against bacterial diseases.
Antimicrobial Activity of Carbapenems
Carbapenems have demonstrated in vitro activity against a broad range of bacteria, including Streptococcus pneumoniae, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii.
Imipenem, the first carbapenem antibiotic to be introduced, is active against most Gram-negative and Gram-positive bacteria. Meropenem is another commonly used carbapenem antibiotic that has demonstrated excellent activity against Enterobacteriaceae, P. aeruginosa, and S. aureus. Ertapenem, a newer carbapenem antibiotic, is primarily used for the treatment of community-acquired infections and has excellent activity against Gram-negative bacteria.
Carbapenems are typically reserved for the treatment of serious infections caused by multidrug-resistant bacteria or infections caused by organisms that are difficult to treat with other antibiotics.
Carbapenem Resistance Mechanism
The resistance mechanisms of carbapenems vary depending on the bacteria. Here are some of the common mechanisms:
- Carbapenemases: Carbapenemases are enzymes produced by bacteria that can break down carbapenems, rendering them ineffective. There are several types of carbapenemases, including KPC, NDM, and OXA.
- Porin loss: Porins are channels in the bacterial outer membrane that allow antibiotics to pass through. Some bacteria can decrease or eliminate the number of porins, making it difficult for carbapenems to enter the cell.
- Efflux pumps: Efflux pumps are proteins that can pump antibiotics out of the bacterial cell, preventing them from reaching their target. Some bacteria can produce efflux pumps that are specific to carbapenems, making them resistant to these antibiotics.
- Target modification: Some bacteria can modify the target of carbapenems, which is the penicillin-binding protein (PBP) in the bacterial cell wall. Modifications to the PBP can reduce the affinity of carbapenems for their target, making them less effective.
- Overproduction of PBPs: Some bacteria can produce an excess of PBPs, which can saturate the available carbapenems, preventing them from effectively inhibiting cell wall synthesis.

In conclusion, the mechanisms of carbapenem resistance are diverse and complex, making it challenging to develop effective treatments for bacterial infections caused by carbapenem-resistant bacteria. It is crucial to use antibiotics judiciously and adhere to proper dosing regimens to prevent the emergence of carbapenem-resistant bacteria.
Adverse effects of Carbapenems
Like all medications, carbapenems can have side effects, although they are generally considered safe and well-tolerated by most people. Some of the most commonly reported side effects of carbapenems include:
- Diarrhea
- Nausea and vomiting
- Headache
- Rash
- Allergic reactions, such as hives or difficulty breathing
- Elevated liver enzymes
- Seizures (in patients with a history of epilepsy or other seizure disorders)
- Prolonged bleeding time (in patients with renal impairment)
These adverse effects are generally minor and go away after the medicine is stopped. However, carbapenems can cause serious side effects such as severe allergic reactions, anaphylaxis, and superinfection with Clostridioides difficile (C. diff) in rare cases.
It is critical to closely monitor carbapenem-treated patients for symptoms of adverse effects and to cease the medicine if any major side effects are noticed.
Warning: This article is intended for educational and research purposes only, not for clinical application.