Cellular biology delves into the intricate ways cells maintain their internal environment, interact with their surroundings, and regulate their functions. Essential to these processes are passive and active transport mechanisms. These systems control the movement of vital substances into and out of cells. This article explores these mechanisms, focusing on tonicity, diffusion, osmosis, and the role of membrane proteins in active transport.
Tonicity and Its Impact on Cells
Tonicity describes how the concentration of solutes in a solution influences water movement across a cell membrane. Understanding tonicity is crucial for grasping how cells maintain their shape and function in various environments.
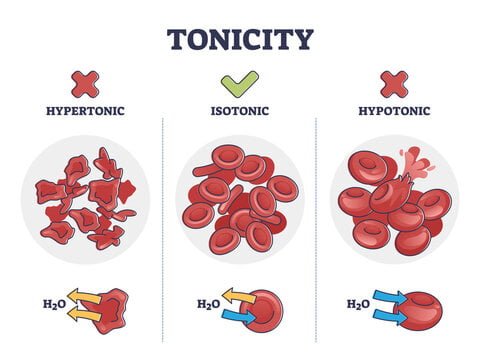
Hypotonic Solutions
A hypotonic solution has a lower solute concentration compared to the inside of the cell, resulting in a higher concentration of water molecules outside the cell. This imbalance affects cells in several ways:
- Osmotic Pressure Gradient: Water moves into the cell due to the osmotic pressure gradient created by the lower solute concentration outside. Osmosis involves water traveling through a semi-permeable membrane from areas of lower solute concentration to higher solute concentration. Although the cell membrane permits water passage, it restricts solute movement.
- Cell Swelling: As water enters the cell, it accumulates in the cytoplasm, causing the cell to expand. This swelling increases internal pressure and can stretch the cell membrane.
- Lysis Risk: Persistent hypotonic conditions can lead to excessive water entry, resulting in internal pressure that may cause the cell to burst or lyse, thus releasing its contents into the extracellular space.
Isotonic Solutions
An isotonic solution matches the solute concentration of the cell’s cytoplasm. The primary characteristics of isotonic solutions include:
- Balanced Osmotic Pressure: In this environment, the osmotic pressure inside and outside the cell is equal. Therefore, water movement into and out of the cell is balanced, with no net change in water volume.
- Stable Cell Volume: Due to the balanced water movement, the cell retains its normal shape and volume. Isotonic solutions are crucial in medical treatments, such as intravenous fluids, to prevent cellular swelling or shrinkage.
Hypertonic Solutions
A hypertonic solution contains a higher concentration of solutes compared to the inside of the cell. The effects on cells include:
- Osmotic Pressure Gradient: Water exits the cell to the hypertonic ECF, driven by the higher solute concentration outside. This movement helps equalize solute concentrations across the membrane.
- Cell Shrinkage: Water loss causes the cell to shrink, a process known as crenation. This shrinkage can impair cell function and, if severe or prolonged, may lead to cellular damage.
- Medical Uses: Hypertonic solutions can be used therapeutically to treat conditions such as edema by drawing excess fluid out of tissues.
Diffusion vs. Osmosis
Osmosis and diffusion are fundamental passive transport mechanisms that involve different substances and processes.
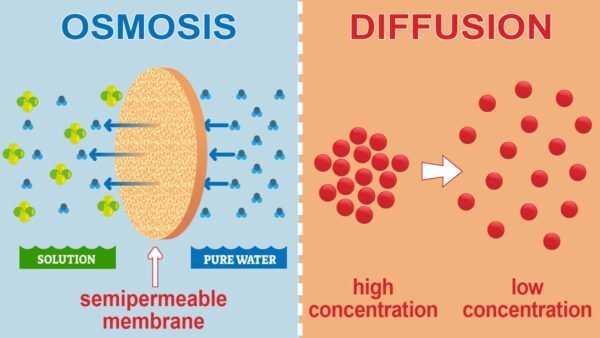
Diffusion
- Definition: The process of Diffusion is the movement of solutes from an area of higher concentration to an area of lower concentration. This process continues until the solute concentration reaches equilibrium.
- Process: Solute particles move freely across the plasma membrane if it is permeable to them. This mechanism does not require energy (ATP). For instance, the spread of a perfume scent in a room demonstrates diffusion.
Osmosis
- Definition: Osmosis is a type of diffusion focused on water movement. It occurs from areas of lower solute concentration (hypotonic) to areas of higher solute concentration (hypertonic) through a semi-permeable membrane.
- Process: Osmosis balances water concentrations on either side of the membrane. In isotonic solutions, water movement is balanced. However, in hypotonic and hypertonic solutions, water adjusts to balance solute concentrations.
Active Transport via Membrane Proteins
Active transport is essential for moving substances across cell membranes against their concentration gradients. Unlike passive transport, which relies on natural gradients, active transport requires energy in the form of adenosine triphosphate (ATP).
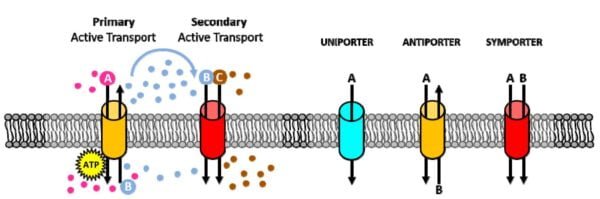
Primary Active Transport
- Sodium-Potassium Pump: This pump is vital for maintaining sodium and potassium ion gradients across the cell membrane. It transports three sodium ions out of the cell and two potassium ions into the cell per ATP molecule hydrolyzed. This process creates essential gradients for various cellular functions, including nerve impulse transmission and muscle contraction.
Secondary Active Transport
- Mechanism: Secondary active transport indirectly utilizes ATP by relying on the gradients established by primary active transport. For example, the sodium gradient generated by the sodium-potassium pump helps drive the transport of substances like glucose against their gradients.
- Co-Transport: This mechanism often involves co-transporters that use the sodium ion movement down its gradient to move other substances against their gradients.
Types of Membrane Pumps
- Uniport Pumps: These transport a single substance in one direction across the membrane. For example, the calcium pump helps maintain low intracellular calcium levels by moving calcium ions out of the cell.
- Symport Pumps: These move two or more substances in the same direction. An example is the sodium-glucose symporter, which transports both sodium ions and glucose into the cell simultaneously.
- Antiport Pumps: These transport substances in opposite directions. The sodium-potassium pump is a classic antiport example, moving sodium ions out and potassium ions into the cell.
Active Transport via Vesicles
When substances are too large or too polar to pass through the plasma membrane directly, cells use vesicular transport.
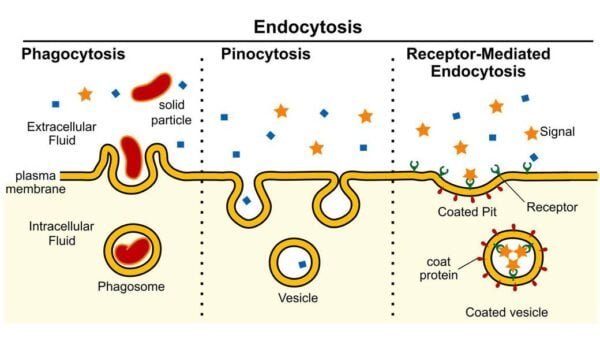
Vesicular Transport
- Vesicles: These are small, membrane-bound sacs that transport large molecules or particles into or out of the cell. Vesicles can either fuse with the plasma membrane to release their contents or form from the membrane to internalize substances.
- Energy Requirement: Vesicular transport is an active process requiring ATP to move vesicles and their contents across cellular membranes.
Endocytosis
- Phagocytosis: Often termed “cell eating,” this process involves the engulfing of large particles such as bacteria or dead cells. The engulfed material is enclosed in a phagosome, which subsequently fuses with lysosomes for digestion.
- Pinocytosis: Known as “cell drinking,” this process involves the intake of fluid droplets from the extracellular space. Small vesicles enclose the fluid, which is then processed inside the cell.
- Receptor-Mediated Endocytosis: This specific type of pinocytosis uses surface receptors to selectively internalize specific molecules, such as hormones or cholesterol. The binding of these molecules to their receptors triggers vesicle formation and internalization.
Exocytosis
- Process: Exocytosis is the reverse of endocytosis. It involves vesicles fusing with the plasma membrane to release their contents outside the cell. This process is vital for the secretion of hormones, neurotransmitters, and other essential molecules.
Conclusion
A comprehensive understanding of passive and active transport mechanisms is fundamental in cellular biology. Passive processes, such as diffusion and osmosis, depend on concentration gradients and do not require energy. Conversely, active transport processes, including primary and secondary transport, utilize energy to move substances against their gradients. Additionally, vesicular transport accommodates molecules too large to pass through the plasma membrane directly.
These transport mechanisms are crucial for maintaining cellular homeostasis, facilitating intercellular communication, and allowing cells to adapt to their environments. Mastering these concepts provides valuable insight into the complex and dynamic nature of cellular life.