Osmosis and diffusion are two major types of passive transport that allow particles to penetrate cell membranes as well as other biological barriers. Diffusion requires the movement of the particles from a higher concentration to a lower concentration until an equilibrium is reached, whereas osmosis requires the shift of water molecules across a semipermeable membrane from a region of lower solute concentration to a region of greater concentration of solute. Both procedures are essential for preserving cells’ optimal functioning and are crucial to several biological and chemical functions.
Understanding Osmosis: 8 Key Points to Know
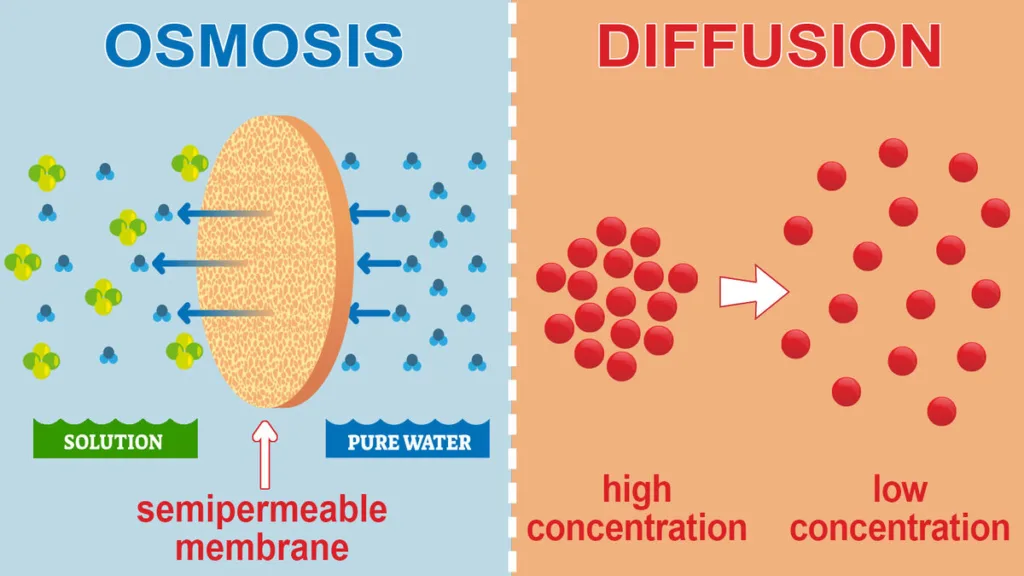
- Osmosis is a sort of passive transport in which solvent molecules (often water) travel over a semipermeable membrane from a region with a lower concentration of solutes to one with a greater concentration.
- The gradient in the concentration of solutes determines the direction of water flow, which moves from the side with lower solute concentration to the side with greater solute concentration until equilibrium is attained.
- Osmosis cannot take place without the semipermeable membrane because it only permits the passage of water molecules while blocking the flow of solute particles.
- Osmosis is essential for preserving the structure and functionality of cells, especially in creatures without cell walls, like animal cells.
- Osmosis is vital for biological activities including nutrition absorbing and waste disposal.
- Osmosis can cause cells to expand or contract based on the relative concentration of solutes both within and outside the cell.
- Osmosis rate is affected by a number of variables, including temperature, membrane permeability, and the gradient of solute concentration.
- Osmosis has several useful uses, including water desalination and purification, food preservation, and the preservation of biological materials.
Factors affecting Rate of Osmosis
- Concentration gradient: The direction and rate of water movement is determined by the difference in solute concentration between the two sides of the membrane.
- Temperature: The rate of osmosis increases with increasing temperature, as molecules move more quickly.
- Pressure: The rate of osmosis can be slowed by increasing pressure on the side with higher solute concentration or increased by reducing pressure.
- Surface area: The rate of osmosis increases with increased surface area of the membrane, as it allows more water to pass through.
- Membrane permeability: If the membrane is more permeable to water, the rate of osmosis is higher when the membrane is more permeable to water.
- Solubility of solutes: The rate of osmosis will increase, if the solutes are more soluble in the membrane.
- Molecular weight: Smaller solutes will diffuse more quickly, causing osmosis to occur at faster rates.
- Distance: Osmosis occurs more quickly the closer the distance is between the two sides of the membrane.
Understanding Diffusion: 8 Key Points to Know
- The movement of particles (atoms, molecules, ions, etc.) from a region of higher concentration to an area of lower concentration is known as diffusion, which is a sort of passive transport.
- The concentration gradient determines the direction of particle motion, which moves from regions with high concentration to regions of low concentration until equilibrium is attained.
- Diffusion may happen in any media, including air, water, and solids, and does not need a barrier membrane to do so.
- The size and charge of the particles, the viscosity of the medium, the temperature, and the existence of barriers or impediments are some of the variables that affect the rate of diffusion.
- Many biological functions, including the absorption of nutrients and the removal of waste, depend on diffusion.
- Due to the higher degree of molecular mobility and absence of intermolecular interactions, diffusion in gases occurs far more quickly than it does in liquids and solids.
- Diffusion is vital for a variety of natural processes, including the dispersion of carbon dioxide and oxygen in the environment, the combining of nutrients in soil, and the exchange of heat and gases within the human body.
Factors affecting Rate of Diffusion
- Concentration gradient: The difference in concentration between the two regions will determine the direction and rate of diffusion.
- Temperature: Higher the temperature, higher the rate of diffusion, as molecules move more quickly.
- Pressure: Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it.
- Molecular weight: Higher rate of diffusion, smaller the molecular weight.
- Medium: The viscosity and density of the medium can influence the rate of diffusion. More viscosity, less diffusion rate.
- Distance: The shorter the distance between the two regions, the faster the rate of diffusion.
- Surface area: Larger the surface area for diffusion, higher the rate of diffusion.
- Barrier permeability: The permeability of barriers, such as cell membranes, can impact the rate of diffusion.
Differences between Osmosis and Diffusion
| Property | Osmosis | Diffusion |
| Definition | The movement of solvent molecules across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration | The movement of particles (atoms, molecules, ions) from an area of higher concentration to an area of lower concentration |
| Type of transport | Passive transport | Passive transport |
| Membrane involved | A semipermeable membrane is required | No membrane is required |
| Direction of movement | From a dilute solution to a concentrated solution | From a higher concentration to a lower concentration |
| Driving force | Concentration gradient of solute particles | Concentration gradient of particles |
| Effect on concentration | Can result in an equalization of solute concentrations on both sides of the membrane | Results in an equalization of particle concentrations throughout the medium |
| Nature of particles involved | Only solvent molecules (usually water) | Any kind of particle (atoms, molecules, ions, etc.) |
| Rate of transport | Slower compared to diffusion | Faster compared to osmosis |
| Energy requirement | Does not require energy | Does not require energy, but may be facilitated by a carrier protein in facilitated diffusion |
| Factors affecting transport | Concentration gradient, temperature, pressure, size of particles | Concentration gradient, temperature, pressure, size and charge of particles, viscosity of medium |
| Effect of external pressure | Can be counteracted by applying external pressure | No significant effect |
| Types of solutions involved | Usually involves solutions with different solute concentrations | Can involve solutions with the same or different solute concentrations |
| Examples | Movement of water into plant cells | Diffusion of oxygen into cells during respiration |
| Importance in biological systems | Critical for maintaining cell shape and function | Important for many cellular processes such as nutrient uptake and waste elimination |
| Applications in industry | Used in water purification and desalination | Used in gas separation and drug delivery |