What is Monosodium glutamate (MSG)?
The sodium salt of the non-essential amino acid glutamic acid, one of the most prevalent amino acids in nature, is known as monosodium glutamate (MSG). Glutamate is thus found in a wide variety of foods, and in its free form has been shown to have a flavour enhancing effect. Due to its ability to enhance flavor, glutamate is frequently added on purpose to food, either in the form of hydrolyzed protein or pure monosodium salt (MSG).
MSG, usually referred to as monosodium glutamate, is a flavor enhancer frequently used in processed food and snacks as well as Asian cuisine. In addition to sweet, salty, sour, and bitter, the umami flavor, which is the fifth fundamental taste sense, is frequently enhanced by the addition of MSG to food. While some people may respond negatively to MSG, the FDA generally considers it to be safe when consumed in moderate amounts. It is significant to note that although regulatory bodies regard MSG to be safe, some people may suffer negative effects such as headaches, flushing, and sweating after consuming it.
History of MSG
A Japanese scientist by the name of Kikunae Ikeda first discovered MSG in 1908 when he became aware of a distinct flavor in seaweed broth. He discovered the ingredient that gave this flavor and named it the Japanese word “umami,” which means “deliciousness” or “savory.”
Professor Ikeda had determined that L-glutamic acid (glutamate), a non-essential amino acid, was responsible for the savory flavor. MSG is a substance that was created by combining salt with glutamate. Later, MSG was created as a product that was added to food as a flavoring.
MSG rapidly became an essential component in many foods in Japan, especially soups and broths. MSG quickly gained popularity as a flavor enhancer in many different cuisines throughout the world once the rest of the world caught on.

Sources of MSG
MSG is made by fermenting sugar beets, sugar cane, or corn, molasses using a bacteria called Corynebacterium glutamicum. After that, the glutamic acid is extracted and given a sodium treatment to make MSG. This fermentation process is similar to that used to make yogurt, vinegar and wine.
Structure of MSG
Monosodium glutamate, also known as MSG, it is a white, crystalline powder having the formula C5H8NO4Na. It is a sodium salt of glutamic acid, an amino acid naturally occurring in a variety of foods such meat, fish, and vegetables. Here are some details on MSG’s composition:
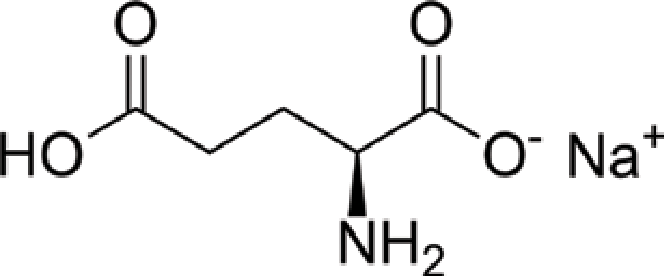
Amino acid structure: A sodium salt of the amino acid glutamic acid is MSG. Proteins are made up of amino acids, which all have a common structural formula that includes a central carbon atom, an amino group (-NH2), a carboxyl group (-COOH), and a side chain or R group.
Glutamic acid: The amino group is attached to the carbon atom next to the carboxyl group in glutamic acid, making it an alpha-amino acid. It has a side chain with an amino group and a carboxylic acid group that can create a cyclic structure.
Sodium ion: The positively charged carboxylate group (-COO-) of the glutamic acid molecule is attracted to the negatively charged sodium ion (Na+) in MSG.
Crystalline form: MSG is a white crystalline powder that is highly soluble in water. Its crystal structure is cubic.
Physical and Chemical properties of MSG
Physical properties
- Appearance: MSG is a white crystalline powder.
- Solubility: MSG is highly soluble in water and slightly soluble in ethanol.
- Melting point: The melting point of MSG is approximately 253-255 °C.
- Odor: MSG is odorless.
- Taste: MSG has a savory, umami taste.
Chemical properties
- Chemical formula: The chemical formula of MSG is C5H8NO4Na.
- Molecular weight: The molecular weight of MSG is approximately 169.11 g/mol.
- Acidity: With a pH of around 6.5 in aqueous solutions, MSG has a mild acidic flavor.
- Stability: At normal pressures and temperatures, MSG is stable and difficult to decompose. When exposed to high temperatures or moisture, it may, still deteriorate over time.
- Reactivity: MSG does not react with most chemicals, although it can react with strong acids and bases.
Production process of Monosodium glutamate (MSG)
The production of MSG can be broken down into several steps:
Fermentation: A carbohydrate source, like molasses, starch, or sugarcane, is first fermented to produce MSG. A bacteria strain known as Corynebacterium glutamicum is introduced into the carbohydrate source after it has been sterilized. Glutamic acid is a by-product of the bacteria’s fermentation of the carbohydrates.
Purification: After the fermentation is finished, the resultant broth is filtered to get rid of any impurities or solids. on exchange chromatography is then used to separate the glutamic acid from the other ingredients in the broth.
Conversion: The purified glutamic acid is then converted into MSG through a chemical process called neutralization.The salt is produced by mixing sodium hydroxide with glutamic acid, crystallizing it, and drying it.
Quality control: To ensure that the MSG is of a consistent quality and purity throughout the production process, strict quality control techniques are implemented. In order to confirm that the product meets the necessary requirements, samples are sent for analysis and the product is examined for contaminants and impurities.
Packaging: Once the product has been tested and approved, it is packaged into containers and shipped to food manufacturers around the world. The packaging is designed to protect the product from moisture and other impurities and to keep it stable throughout storage and transportation.
Overall, the manufacture of MSG is a complex procedure that includes meticulous fermentation, purification, conversion, and quality control. The final product is a high-quality taste enhancer that is frequently utilized in the food industry.
Effects of MSG
Research and controversy surround the effects of MSG (monosodium glutamate) on the human body. Here are some points outlining some of the effects that have been studied:
Flavor enhancer: MSG is frequently added to processed food, soups, and food snacks to improve the flavor. It activates the umami receptors on the tongue, which inform the brain that the food is sweeter and more delicious.
Adverse reactions: Headaches, excessive sweating, and nausea are just a few of the alleged negative effects that some individuals claim to suffer after consuming foods that contain MSG. This phenomenon is known as Chinese Restaurant Syndrome (CRS), although the existence of this syndrome is still debated in the scientific community.
Asthma: High MSG consumption has been linked in certain studies to an increased risk of asthma symptoms, although further study is required to confirm this association.
Obesity: Consuming food rich in MSG may be linked to an increased risk of obesity, while the exact mechanism behind the connection is not completely understood.
Blood pressure: Although additional study is needed to confirm this connection, several studies have revealed that eating foods rich in MSG may raise the risk of high blood pressure.
Is MSG safe to eat?
Yes, most people believe that it is safe to consume monosodium glutamate (MSG). MSG is a flavoring agent that is frequently used in a variety of cuisines, particularly Asian food preparation. FDA considers the addition of MSG to foods to be “generally recognized as safe” (GRAS).
MSG’s safety has been thoroughly investigated, and regulatory agencies from all over the world, including the U.S. Food and Drug Administration (FDA), have determined that it is safe to consume in moderation. However, some people who consume MSG may develop moderate adverse effects like headaches, sweating, and nausea. However, these side effects are rare, and most people can consume MSG without any adverse effects.
We may examine the ingredient list on food labels to verify if a certain product has MSG if we have concerns about consuming it. We might also make an effort to eat less processed foods as they are more likely to contain MSG.
How can we know if there is MSG in foods?
MSG must be included as an ingredient on food labels in the United States under FDA regulations. It could be referred to on the label by its common name, “monosodium glutamate,” or by a more specific word, such “flavor enhancer” or “natural flavoring. t’s a good idea to check the ingredients list on the label if we’re not sure if a food product includes MSG.
According to FDA regulations, Monosodium glutamate must be listed as an ingredient in the ingredient label on items that have added MSG. But MSG may be found in foods naturally in items like tomatoes and cheeses, as well as in hydrolyzed vegetable protein, autolyzed yeast, hydrolyzed yeast, yeast extract, and soy extracts.
While the FDA requires that these goods’ ingredients be mentioned on the ingredient label, it does not require that their labels indicate whether or not MSG is present naturally in the product. Foods, however, cannot make the claim that they include “No MSG” or “No added MSG” on their package if they contain any component that naturally contains MSG.MSG also cannot be listed as “spices and flavoring.”
In conclusion, research and discussion about the effects of MSG on the human body are currently continuing. While MSG is usually regarded ineffective for most people when consumed in moderation, certain individuals may be more susceptible to its effects than others. It’s essential to take MSG in moderation and to be aware of any potential negative side effects, just as with any other dietary ingredient.
Learn More
Reference
- https://www.foodstandards.gov.au/publications/documents/MSG%20Technical%20Report.pdf
- https://www.britannica.com/science/monosodium-glutamate
- https://ajps.journals.ekb.eg/article_187828_1481015fa488e5706689b06df4a4aac6.pdf
- https://www.fda.gov/food/food-additives-petitions/questions-and-answers-monosodium-glutamate-msg