What is Acid insoluble ash?
When a sample of food is ignited at high temperatures and then treated with acid to dissolve the soluble minerals, the resulting inorganic residue is known as acid insoluble ash. The Acid Insoluble Ash (AIA) in Food Analysis is made up of minerals like silica, sand, and other types of minerals that are difficult for acid to dissolve in or break down. These materials are not easily soluble in acid.
The proportion of acid-insoluble ash in a food sample is frequently used as an indicator for the presence of contaminants like sand, soil, and other foreign materials. High quantities of acid-insoluble ash may indicate contaminated food, such as heavy metals or pesticide residues, as well as poor food quality.

Principle of Acid Insoluble Ash (AIA) in Food Analysis
The main objective of the acid-insoluble ash term is to determine the quantity of inorganic residue in a sample of food that is not soluble in acid. The quantity of extraneous material, such as sand, dirt, or other impurities that may be present in the sample, can be determined using the acid-insoluble ash content, which serves as a measure of the food sample’s purity.
In the acid-insoluble ash method, the food sample is incinerated to eliminate any organic matter before hydrochloric acid is used to dissolve the soluble minerals in the remaining ash. The acid-insoluble residue is then collected and weighed on filter paper. A percentage of the initial sample weight is used to represent the weight of the acid-insoluble residue.
The basic concept behind this principle is that the acid-insoluble residue is made up of minerals like silica, sand, and other types of minerals that are not easily dissolved in acid. It is possible to determine the number of impurities and contaminants present in a food sample by measuring the amount of acid-insoluble ash in the sample.
In the food industry, the acid-insoluble ash technique is frequently used to evaluate the quality and purity of food items including grains, animal feed, and other agricultural goods. The method is also used in the analysis of soil samples to determine the mineral content of the soil.
Apparatus Required
- Balance machine
- Muffle furnace
- Hot air oven
- Desiccator
- Hot plate
- Porcelain crucible
- Heat proof gloves
- Spatula
- Tong
- Ashless filter paper
- Measuring cylinder
- Dropper
- Hydrochloric acid
Procedure of Determination of Acid Insoluble Ash
Step -1
Chemical preparation
1. 40% HCL preparation
- By diluting 40ml HCL (concentrated) in 60ml of water to make the total volume of 100ml.
- Store in glass bottle in dark and cold place.
Step -2
Sample Incineration
- Bring a clean porcelain crucible.
- Pace the crucible on balance machine and tare its weight.
- Take around 2g of the sample to the crucible, and then note the sample weight.
- Place the crucible inside the muffle furnace and cover it with the lid.
- Burn the sample for 30 minutes at 550° C
Ashing of sample
- Take the crucible out from muffle furnace after it has burned for 30 minutes. Once it has cooled, remove the lid, and carefully examine the ash.
- Add few drops of distilled water to the ash content.
- Development of black color indicates that the ash hasn’t become carbon free yet.
- If black color may appear again burn at 550 °C until it becomes carbon free.
Step-3
Boiling in Acid and Filtration
- Measure 25ml of 40% hydrochloric acid solution and pour the solution into the crucible containing ash.
- Place the crucible on the hot plate and boil the ash content in the HCL solution for 5 min.
- After 5 min of boiling, remove the crucible from the hot plate by using a tong.
- Now, prepare a filter by using an ashless filter paper and immediately filter the ash solution while it is still warm.
- The few particles are trapped on the filter paper and these particles will be counted as Acid Insoluble Ash.
- Wash the crucible with hot water using the same filter paper.
- Add additional hot water to the filter paper washing it to ensure that no residue of HCL is left with the filter paper.
Step-4
Crucible Preparation
- Place the crucible and lid inside the hot air oven at 110 °C for 30min.
- After drying for 30 minutes, cool it in a desiccator.
- After cooling for 10 minutes, weigh the dry blank crucible and note the weight.
Step-5
Filtrate Incineration
- Fold the filter paper carefully to avoid the loss of the filtrate.
- Place the folded paper into the prepared crucible and cover the crucible with a lid.
- Now, place the crucible inside the furnace at 550°C for 90min.
Ashing of filtrate
- After 90min of burning, take out the crucible from the furnace after cooling.
- Take the weight of crucible containing acid insoluble ash.
- Now, note the weight for using it in the calculation.
Step-6
Calculation
Acid Insoluble Ash=
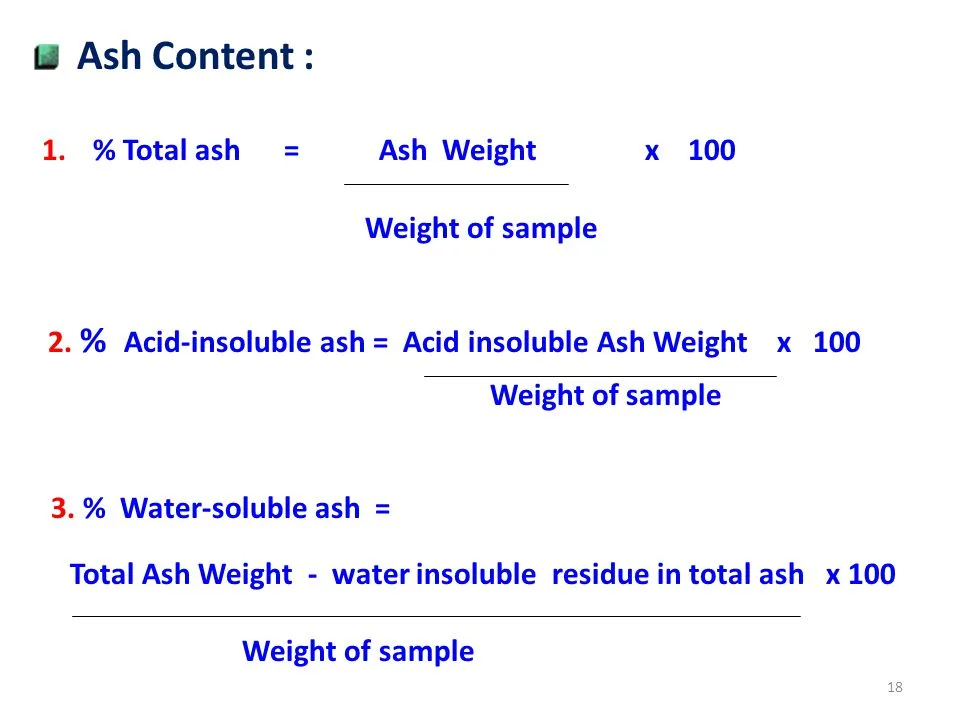
Where,
- Wf is crucible + Ash weight
- Ws is sample weight
- Wc is crucible weight
Application of Acid Insoluble Ash determination
The acid insoluble ash has several applications in food industry, including:
Quality control: The purity and quality of food products, including cereals, animal feed, and other agricultural goods, are determined by using the acid-insoluble ash content as a quality control standard. The presence of contaminants and impurities in the sample may be indicated by high amounts of acid-insoluble ash.
Nutritional analysis: In nutritional analysis, the acid-insoluble ash content is used to estimate the mineral content of food products. Minerals like calcium, phosphorus, and magnesium that are essential to both human and animal nutrition may be determined from the total amount of acid-insoluble ash.
Soil analysis: The acid-insoluble ash method is used in the analysis of soil samples to determine the mineral content of the soil. It is possible to determine the mineral content of the soil, which is essential for agricultural reasons, by measuring the quantity of acid-insoluble ash in a soil sample.
Contaminant analysis: The acid-insoluble ash method may be used for analyzing food samples for contaminants like heavy metals. High concentrations of acid-insoluble ash might be a sign that the sample contains contaminants.
https://thesciencenotes.com/category/food-science/