Saponification value is defined as the number of milligrams of KOH required to completely hydrolyze (saponify) one gram of the oil/fat.
Saponification is a chemical reaction that occurs when fats and oils are hydrolyzed (or broken down) in the presence of an alkali, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH). It is frequently used in the production of soap.
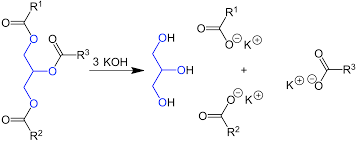
Principle
The main objective of the saponification test for oil or fats in a food sample is to determine the presence and amount of fats or oils in the sample. This test helps assess the quality and composition of the food product, particularly regarding its fat content.
The saponification value test involves converting the fats or oils present in the food sample into soap through the saponification process.
Apparatus required
- Balance machine
- Burette with stand
- Reflux condenser
- Heating mantle
- Distillation unit
Chemical and reagents
- Potassium hydroxide
- Absolute ethanol
- Phenolphthalein
- Hydrochloric acid
- Aluminum foil
Chemical preparation
Step-1
4% Ethanolic KOH
- Weight 1.67g potassium hydroxide pellets.
- Take the potassium hydroxide pellets into distillation flask.
- Now, weight 1g aluminum foil.
- Transfer the aluminum foil into the same distillation flask.
- Measure 200ml absolute ethanol and pour into the same flask.
- Attach a reflux condenser with the flask.
- Now, heat the flask and reflux the alcohol with KOH and Al foil for 30min.
- After 30min of reflux, remove the reflux condenser and set a distillation unit.
- Distil and collect 180ml ethanol after discarding first 10ml.
- Now, turn off the heating of the distillation unit.
- Now, label the collected Ethanol flask.
- Bring a clean mortar and pestle and KOH pellets.
- Take some KOH pellets in the mortar and grind the pellets with pestle.
- Now, take weight of 6g of KOH pellets.
- Transfer KOH pellets in the flask to which 4% Ethanolic KOH solution will be prepared.
- Now, measure 150ml ethanol which were collected from the distillation.
- Pour the ethanol into flask containing KOH pellets.
- Mix and dissolve the KOH in ethanol, keeping the flask into cold water.
- Now, 4% alcoholic potassium hydroxide solution is prepared.
0.5N Hydrochloric Acid
- Dilute 4.1ml of HCL (concentrated) with distilled water to make the total volume of 100ml.
- Standardize newly prepared 0.5N HCL with standard NaOH solution and find the actual normality.
Phenolphthalein indicator
Dissolve 2g phenolphthalein indicator powder into 100ml of ethanol and mix well by shaking.
Step 2
Sample and blank preparation
- Bring two flasks of 250ml for sample and blank preparation.
- Take approx. 5g of oil or fats in the flask labelled with sample.
- Note the sample weight.
- Now, measure 50ml of 4% ethanolic KOH and pour into the sample flask and Sample is prepared.
- Again, measure 50ml of 4% ethanolic KOH and pour into the blank flask.
- Blank flask contains no sample but it contains only 4% ethanolic KOH.
- Now, sample and blank are ready to go the next step.
Step-3
Reflux sample
- Prepare heating mantle.
- Place the sample flask on the heating mantle carefully.
- Now, attach a condenser with the sample flask.
- Heat the sample flask at the boiling points for 30min.
- Turn on the cold-water flow through the condenser.
- After 30 min of reflux, pull up the flask and check it for the separated oil layer.
- Transparent mixture indicates the end of the saponification.
- Now, cool the flask and remove the condenser.
- (If any separated oil is seen, continue saponification for another 15min. In that case, blank should be reflux for 15min more as done for sample).
Reflux blank
- In the same way, boil and reflux the blank for 30 min.
- After 30 min of reflux, stop heating, cool the blank flask and remove the condenser.
Step-4
Titration of sample
- After saponification, bring the cooled sample flask for titration.
- Add a few drops of phenolphthalein indicator solution in the flask.
- Shake the flask for the proper mixing.
- Take 0.5N HCL solution in the burette.
- Note the initial burette reading for sample titration.
- Start titration using 0.5N HCL solution.
- Titration should be carried out with vigorous agitation of the flask.
- Disappearance of pink colour indicated the end points of titration.
- Shake the flask well and add few more drops of 0.5N HCL if needed.
- Stop titration when the pink colour is disappeared completely.
- Note the final reading for sample titration.
Titration for blank
- Blank should be reflux for the time needed to saponify the sample completely.
- Bring the cooled blank flask for titration.
- Add a few drops of phenolphthalein indicator solution into the flask.
- Shake the flask for proper mixing.
- Take 0.5N HCL solution in a burette.
- Note the initial burette reading for blank titration.
- Start titration using 0.5N HCL solution.
- Titration should be carried out with vigorous agitation of the flask.
- Disappearance of pink colour indicated the end points of titration.
- Shake the flask well and add few more drops of 0.5N HCL if needed.
- Stop titration when the pink colour is disappeared completely.
- Note the final reading for sample titration.
Step -5
Calculation
Saponification value =
Where,
- VS = for sample
- VB = for blank
- WS= weight of sample.

Application of saponification value in fats and oil
Determining Fat Content:
The saponification test can quantify fats and oils in food samples. The amount of soap generated after converting the fats and oils into soap can be used to determine the original fat content of the sample. This information is essential for nutritional labeling and quality control.
Quality Assessment:
Saponification can be used to evaluate the quality and authenticity of food items. Different fats and oils have different saponification values, and by comparing the saponification values of the sample to established standards, the authenticity or potential adulteration of the product may be determined.
Analysis of Lipid Composition:
Saponification is frequently used as a first step in the study of lipid content in food samples. After saponification, the resultant soap can be examined further using methods such as gas chromatography or mass spectrometry to identify and quantify individual fatty acids in the sample.
Removal of Undesirable Components:
Unwanted Component Removal: Saponification can be used to eliminate some undesired components from food samples. The saponification process, for example, can aid in the removal of certain impurities or contaminants found in fats and oils, such as free fatty acids or rancid compounds, resulting in a cleaner and purer product.
Soap Production:
Saponification is also utilized in the making of soap, which is not directly connected to food analysis. As a byproduct, certain food producers may create soaps made from food-grade fats and oils.
References:
- Association of Official Analytical Chemists (AOAC). (2016). AOAC Official Method 966.06: Fat (Total, Saturated, and Unsaturated) in Foods. AOAC International.
- Fox, J.B., and others. (2010). Food Analysis Laboratory Manual. Springer Science & Business Media.
- Gamlath, C.B. (2017). Fats and Fatty Acids in Human Nutrition: A Literature Review. Ceylon Medical Journal, 62(1), 25-30.
- Thirumalesh, B.V., and Sashikala, V.B. (2019). Determination of Saponification Value of Different Oils for Biodiesel Production. International Journal of Chemical Studies, 7(2), 1910-1912.
Learn more