By examining the movement of solutes like potassium iodide and glucose, and tracking weight changes in varying sucrose concentrations, the experiment aims to demonstrate the principles of passive transport and how concentration gradients impact molecular movement across membranes.
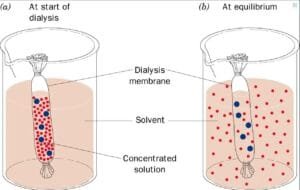
This experiment investigates how varying concentrations of solutes influence the processes of osmosis and diffusion across a semipermeable membrane (dialysis tubing), examining the movement of water, solutes, and the resulting changes in solution characteristics and weight over time.
Introduction
Osmosis and diffusion are fundamental processes in cellular transport, playing important roles in maintaining homeostasis and enabling essential biochemical functions (Alberts et al., 2014). Both are forms of passive transport, allowing molecules to move across biological membranes without energy. Osmosis is defined as the movement of water molecules through a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration until equilibrium is reached. In contrast, diffusion involves the movement of solute particles from an area of higher concentration to an area of lower concentration (Binod, 2024).
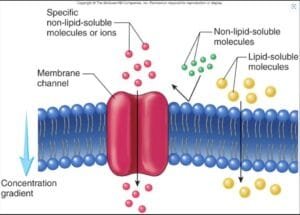
Understanding the dynamics of these processes is necessary for exploring how cells interact with their environment, absorb nutrients, and eliminate waste. There are three main types of solutions when it comes to osmosis: hypertonic, where there are more solutes outside the cell than inside, causing water to flow out of the cell; hypotonic, where there are fewer solutes outside the cell, leading to water moving into the cell; and isotonic, where the concentration of solutes is the same inside and outside the cell, resulting in no net movement of water (Binod, 2024).
In this experiment, Dialysis bags are used in the experiment as they are semipermeable and represent the artificial cells to study the osmosis and diffusion. The hypothesis of the experiment is that the starch/glucose solution within the dialysis tubing will exhibit a color change due to the diffusion of potassium iodide. And the weight of the dialysis bags will change due to osmosis, depending on the sucrose concentrations.
Materials and Methods
Steps for Studying Diffusion and Osmosis Using Dialysis Tubing
Diffusion Experiment:
- Prepare the Diffusion Solution:
- Fill a small beaker with water.
- Add 10 drops of potassium iodide solution to the beaker to achieve a medium brown color.
- Observe Diffusion:
- Record the initial color and appearance of the potassium iodide solution in the beaker.
- Wait for the solution to diffuse, observing the color change until the solution becomes yellow throughout, indicating complete diffusion.
- Prepare Dialysis Tubing:
- Cut a piece of dialysis tubing approximately 10 cm long.
- Clamp one end of the tubing securely and leave the other end open.
- Fill the Dialysis Tubing:
- Fill the open end of the dialysis tubing about two-thirds full with a starch/glucose solution.
- Securely clamp the open end of the tubing.
- Record Initial Observations:
- Record the color of both the potassium iodide solution in the beaker and the starch/glucose solution in the dialysis tubing in Table 1.
- Dip a glucose test strip into the beaker solution and record the result in Table 2.
- Place Dialysis Tubing in Beaker:
- Submerge the prepared dialysis tubing in the beaker containing the potassium iodide solution.
- Wait and Observe:
- After 30 minutes, record the color changes of both the potassium iodide solution in the beaker and the starch/glucose solution inside the dialysis tubing in Table 1.
- Dip the glucose test strip into the beaker solution again and record the result in Table 2.
Osmosis Experiment:
After 60 minutes, record the final weights of all dialysis cells in Table 3.
Prepare Osmosis Solutions:
- Fill a small beaker about two-thirds full with a 25% sucrose solution.
- Fill a large beaker about two-thirds full of a 1% sucrose solution.
Prepare Dialysis Tubing for Artificial Cells:
- Obtain four pieces of soaked dialysis tubing.
- Clip one end of each piece of dialysis tubing with a clip. Label the clips A, B, C, and D.
Fill Dialysis Tubing:
- Open “Cell A” and fill it about two-thirds full with a 1% sucrose solution, then securely clamp it.
- Fill “Cell B” with 1% sucrose solution, “Cell C” with 10% sucrose solution, and “Cell D” with 25% sucrose solution, securely clamping each.
Record Initial Weights:
- Weigh each of the four dialysis cells and record their initial weights in Table 3.
Submerge Dialysis Cells:
- Place “Cell A” in the small beaker with the 25% sucrose solution.
- Place “Cells B, C, and D” in the large beaker with the 1% sucrose solution.
Wait and Weigh:
- After 15 minutes, remove the cells from their respective beakers, dry them slightly, and weigh them.
- Record the new weight in Table 3.
- Return the cells to their respective beakers.
Repeat Weighing Process:
- Repeat the process of removing, drying, weighing, and recording the weight after 30 minutes, 45 minutes, and 60 minutes.
Final Weights:
- After 60 minutes, record the final weights of all dialysis cells in Table 3.

Results
The results of the experiment demonstrate the diffusion of potassium iodide and the behavior of glucose within the dialysis tubing, as shown in the following tables.
Table 1. Diffusion of Potassium iodine solution from beaker to starch/glucose solution from Dialysis tubing
| Potassium iodide solution | Starch/Glucose solution | |
| Beginning color | Yellowish brown | white |
| Ending color | clear | Purple/cloudy |
Table 2. Glucose strip test for diffusion
| Color | Glucose Present? | ||||
| Glucose test strip at beginning | teal | negative | |||
| Glucose test strip at end | Brown/green | Trace of glucose | |||
The results of the osmosis experiment are demonstrated by the movement of solutes across the dialysis tubing and the changes in weight, indicating the hypertonic, hypotonic, or isotonic nature of the environment.
Table 3. Change in weight of Dialysis cells as a Function of time
| 0 minutes (initial weight) (gm) | 10 minutes weight (gm) | 20 minutes weight (gm) | 30 minutes weight (gm) | 40 minutes weight (gm) | |
| Cell A | 24.16 | 24.53 | 23.90 | 24.12 | 24.65 |
| Cell B | 19.27 | 19.31 | 19.34 | 19.30 | 19.33 |
| Cell C | 24.67 | 24.58 | 24.33 | 24.01 | 23.79 |
| Cell D | 28.50 | 26.87 | 25.65 | 25.57 | 24.78 |
Discussion
The experiment demonstrated that potassium iodide diffused into the starch/glucose solution within the dialysis tubing, causing a color change and confirming successful diffusion. Additionally, the weight of the dialysis bags varied based on their surrounding sucrose concentrations, indicating the effects of osmosis: cells in hypertonic solutions lost weight, while those in isotonic solutions remained stable.
Initial observations revealed that the potassium iodide solution started as a yellowish brown, while the starch/glucose solution was white. After 30 minutes, the potassium iodide solution
became clear, and the starch/glucose solution turned purple/cloudy, indicating successful diffusion of the iodine into the tubing.
The glucose test strip initially showed a teal color, indicating no glucose presence. At the end of the experiment, the strip changed to brown/green, confirming a trace of glucose in the beaker solution.
The weight changes over time indicate varying responses to the surrounding solutions. Cell A showed a slight increase in weight after 10 minutes but fluctuated thereafter indicating hypertonic environment in the beaker. Cell B remained relatively stable indicating an isotonic environment. In contrast, Cell C displayed a gradual decrease in weight, while Cell D exhibited a significant decline, reflecting water loss due to its hypertonic environment. The solute moves from higher concentration to lower concentration to maintain homeostasis (Alberts et al., 2014).
The experiment had weaknesses, such as differences in how well the dialysis tubing allowed substances to pass through, which could lead to uneven results. Also, not controlling the temperature and relying on color changes for measurements might have made the findings less reliable.
Overall, the results support the hypothesis that diffusion occurs across the dialysis membrane and highlight the effects of osmotic pressure on the weight of the dialysis bags.
Further experiments
Future experiments could examine at how temperature affects osmosis and diffusion, expecting that higher temperatures will make these processes happen faster. We could also try different substances, like salt or sugar, to see how they change the way molecules move.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2014). Molecular Biology of the Cell (6th ed.). Garland Science.
G.C., Binod. “Osmosis and Diffusion: Differences and Factors That Affect Them.” The Science Notes, 14 Apr. 2023. Web. 2 Oct. 2024. Osmosis and Diffusion: Differences and Factors Affecting Them
Mika, T. A., Klein, R. J., Bullerjahn, A. E., Connour, R. L., Swimmer, L. M., White, R. E.,
Gosses, M. W., Carter, T. E., Andrews, A. M., Maier, J. L., & Sidiq, F. (Eds.). (2024). Anatomy and physiology BIO 211 laboratory manual (3rd ed.). Owens Community College.
G.C., Binod. “Cellular Transport: Passive and Active Mechanisms.” The Science Notes, 3 Sept. 2024. Web. 2 Oct. 2024. Cellular Transport: Passive and Active Mechanisms – The Science Notes