Innate immunity is the body’s first line of defense against pathogens, providing immediate, non-specific protection against a broad range of invaders. Unlike adaptive immunity, which targets specific pathogens with tailored responses, innate immunity acts quickly and uniformly upon encountering threats. This comprehensive defense system includes various external barriers, such as the skin and mucous membranes, and internal mechanisms like phagocytic cells, acute-phase reactants, and inflammatory responses. By understanding the key characteristics and functions of innate immunity, we gain valuable insights into how our body combats infections and maintains health from the very first point of contact with harmful agents.
Understanding Innate Immunity
1. Characteristics of Innate Immunity
- Broad Defense Mechanism: Innate immunity provides a non-specific defense against a wide range of pathogens. It is the body’s initial response system, ready to act immediately upon detecting an invader.
- No Need for Prior Exposure: The innate immune system does not require prior exposure to a pathogen to mount a defense. This is in contrast to the adaptive immune system, which builds specific responses based on previous encounters with pathogens.
- Consistent Response: The innate immune response is uniform, meaning that it provides a similar defensive response regardless of the number of exposures to a pathogen.
External Defense System
The external defense system is the body’s first line of defense against pathogens. It encompasses physical, chemical, and biological barriers designed to prevent infectious agents from entering the body. Here’s a detailed overview of these protective mechanisms:
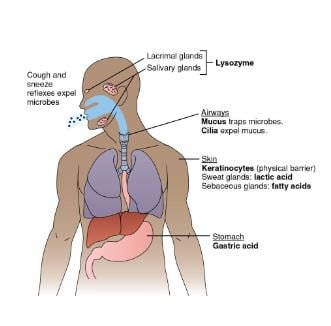
1. Physical Barriers
Skin: The Primary Barrier
- Structure and Function: The skin acts as a formidable physical barrier to pathogen entry. It consists of two main layers:
- Epidermis: The outermost layer is composed of tightly packed epithelial cells covered in keratin, a tough, protective protein that helps prevent pathogen penetration.
- Dermis: Located beneath the epidermis, the dermis contains connective tissue, blood vessels, hair follicles, sebaceous glands, sweat glands, and white blood cells (WBCs). This layer supports the epidermis and contributes to immune defense through its cellular and glandular components.
- Secretions and Chemical Defense: The skin’s secretions play a crucial role in discouraging microbial growth:
- pH Maintenance: The skin’s surface is slightly acidic, with a pH of approximately 5.6. This acidity is maintained by lactic acid and fatty acids secreted by sebaceous glands, creating an environment that inhibits the growth of many pathogens.
- Antibacterial Proteins: Proteins such as psoriasin are present on the skin and have antibacterial effects. Psoriasin specifically targets Gram-negative bacteria like Pseudomonas aeruginosa and Gram-positive bacteria such as Staphylococcus aureus, helping to control bacterial colonization.
2. Mucous Membranes
Function and Location
- Respiratory, Digestive, and Genitourinary Tracts: Mucous membranes line these tracts and serve as a physical barrier to pathogens. They are coated with mucus, a sticky substance that traps and helps to remove pathogens.
- Mucous Secretions: The mucus contains surfactants that prevent bacteria from adhering to epithelial cells, reducing the risk of infection.
3. Specific Defense Mechanisms in Various Tracts
Respiratory Tract
- Cilia: Tiny hair-like structures called cilia line the nasopharyngeal passages and help to clear deposited particles and microorganisms.
- Reflex Actions: Coughing and sneezing are reflexes that expel pathogens from the respiratory tract, helping to prevent their entry into the lower respiratory system.
Digestive Tract
- Stomach Acid: The stomach secretes hydrochloric acid, creating a highly acidic environment with a pH as low as 1. This acidity is effective in killing most microorganisms that enter with food or drink.
- Lysozyme: Found in tears and saliva, lysozyme is an enzyme that attacks the cell walls of Gram-positive bacteria, contributing to the antimicrobial defense of the digestive tract.
Genitourinary Tract
- Urine: The process of urination helps to flush out pathogens from the urinary tract, reducing the likelihood of infection.
- Vaginal pH: In females, the vaginal environment is maintained at a pH of around 5 due to the presence of lactic acid. This acidic pH helps to inhibit the growth of potentially harmful bacteria and yeast.
4. Microbiota (Normal Flora)
Role and Importance
- Competitive Exclusion: The normal flora, or microbiota, consists of various microorganisms that naturally reside on and within the body. These beneficial microbes compete with pathogenic organisms for resources and space, effectively preventing pathogens from establishing themselves.
- Immune System Support: The microbiota also plays a role in modulating the immune response and maintaining overall health, making it a critical component of the external defense system.
Internal Defenses
The internal defense system is crucial for protecting the body from pathogens that manage to bypass external barriers. This system includes a variety of mechanisms and cells that work together to identify, attack, and eliminate intruders. Here’s a comprehensive overview of the internal defense mechanisms:
1. Phagocytic Cells
Role and Function
- Neutrophils: These are the most abundant type of white blood cells and often the first responders to infection. Neutrophils phagocytose (engulf and digest) bacteria and other foreign particles. They are particularly effective in the early stages of infection and are a key component of the innate immune response.
- Macrophages: These cells are derived from monocytes and reside in tissues throughout the body. Macrophages are highly versatile; they not only engulf and destroy pathogens but also play a crucial role in presenting antigens to T cells, thus linking the innate and adaptive immune systems. They help to clear debris from dead cells and aid in tissue repair.
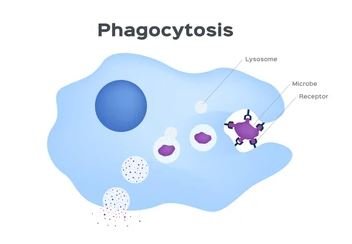
Mechanisms of Microbial Killing
- Oxygen-Dependent Mechanisms: After phagocytosis, neutrophils and macrophages generate reactive oxygen species (ROS) through processes like the respiratory burst. ROS are highly reactive molecules that destroy pathogens by damaging their cellular components.
- Oxygen-Independent Mechanisms: These include the use of antimicrobial peptides, enzymes (e.g., lysozyme), and other molecules that can directly kill or inhibit the growth of pathogens without the need for oxygen.
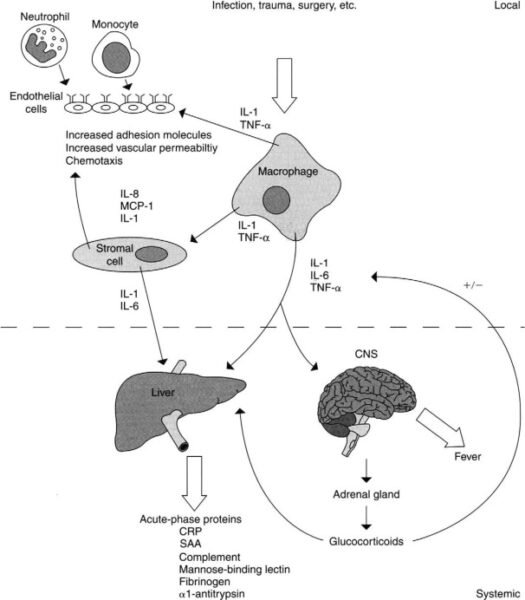
2. Acute-Phase Reactants
Function and Role
- C-Reactive Protein (CRP): One of the key acute-phase reactants, CRP levels increase significantly in response to infection, inflammation, or injury. CRP binds to pathogens and damaged cells, enhancing their recognition and phagocytosis by immune cells.
- Other Acute-Phase Proteins: These include serum amyloid A (SAA) and fibrinogen. These proteins help to further enhance the inflammatory response, contribute to the clotting process, and support the overall immune defense.
3. Protective Reflexes
Description and Function
- Coughing and Sneezing: These reflex actions help to expel pathogens from the respiratory tract. They are triggered by irritation or the presence of foreign particles, and they serve to clear the airways and reduce the risk of infection.
- Vomiting and Diarrhea: In the digestive tract, vomiting and diarrhea are mechanisms to expel ingested pathogens and toxins. Although uncomfortable, these processes help to eliminate potential threats from the gastrointestinal system.
4. Inflammatory Response
Characteristics and Process
- Vasodilation: Inflammation begins with the dilation of blood vessels, which increases blood flow to the affected area. This process brings more immune cells, nutrients, and oxygen to the site of infection or injury.
- Increased Vascular Permeability: The blood vessels become more permeable, allowing immune cells and proteins to pass through and enter the tissues. This helps to isolate and contain the infection.
- Immune Cell Migration: Neutrophils and macrophages migrate from the bloodstream to the site of infection or damage. They perform phagocytosis, engulfing and destroying pathogens, and also release cytokines that help regulate the immune response.
- Resolution: After the threat is eliminated, the inflammatory response subsides, and healing begins. Anti-inflammatory signals are released to repair the tissue and return the area to normal function.
5. Pattern Recognition Receptors (PRRs)
Function
- Recognition of PAMPs: PRRs are specialized receptors on the surface of host cells that detect pathogen-associated molecular patterns (PAMPs). PAMPs are molecules unique to pathogens, such as lipopolysaccharides on bacterial surfaces or viral RNA. By recognizing these patterns, PRRs enable the immune system to distinguish between self and non-self, initiating an appropriate response.
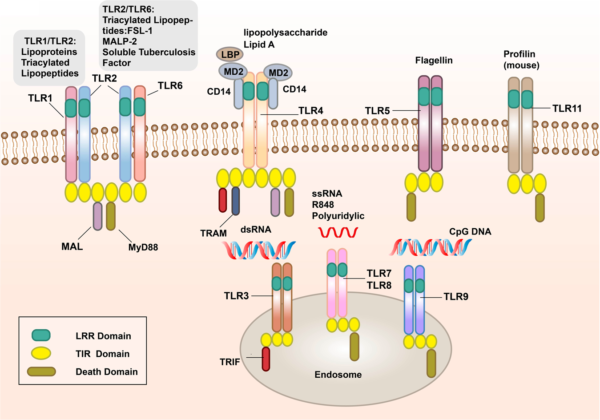
Types of PRRs and Their Functions
- Toll-Like Receptors (TLRs): TLRs are one of the most well-characterized families of PRRs. They are located on the surface of immune cells and within intracellular compartments. Each TLR is specific to different types of PAMPs. For example:
- TLR4 recognizes LPS from Gram-negative bacteria.
- TLR3 detects double-stranded viral RNA.
- TLR5 binds to flagellin, a component of bacterial flagella.
- NOD-Like Receptors (NLRs): NLRs are cytoplasmic PRRs that recognize intracellular PAMPs. They play a role in detecting bacterial peptidoglycans and other pathogen-derived molecules inside the cell. NLRs are involved in initiating inflammatory responses and forming inflammasomes, which are multiprotein complexes that activate inflammatory cytokines.
- RIG-I-Like Receptors (RLRs): RLRs are cytoplasmic receptors that detect viral RNA. They are crucial for recognizing RNA viruses and initiating antiviral responses through the production of interferons and other cytokines.
- C-Type Lectin Receptors (CLRs): CLRs recognize carbohydrate structures on the surfaces of pathogens, such as fungal cell walls. They are involved in the recognition and phagocytosis of fungi and some bacteria.
Activation and Response
- Signal Transduction: Upon recognition of PAMPs, PRRs activate intracellular signaling pathways that lead to the production of pro-inflammatory cytokines and chemokines. These signaling pathways include nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which drive the expression of genes involved in inflammation and immune responses.
- Inflammatory and Immune Response: The activation of PRRs leads to an inflammatory response aimed at eliminating the pathogen. This includes the recruitment of additional immune cells to the site of infection, the activation of antimicrobial mechanisms, and the initiation of repair processes.
What Are Innate Lymphoid Cells (ILCs)?
1. Overview of ILCs
- Origin and Development: Innate Lymphoid Cells (ILCs) originate from the common lymphoid progenitor (CLP) in the bone marrow. They do not express the typical lymphoid markers found on B and T lymphocytes, which makes them distinct from other lymphoid cells.
- Primary Locations: ILCs are predominantly located at mucosal sites throughout the body, including the gastrointestinal tract, respiratory tract, and oral mucosa. These locations are strategic as they are the first line of defense against environmental pathogens.
- Comparison with T Cells: Unlike T cells, which possess specific antigen receptors for targeted immune responses, ILCs lack these receptors. Instead, ILCs sense and respond to signals from tissues that have been compromised by injury or infection, providing a rapid, generalized response.
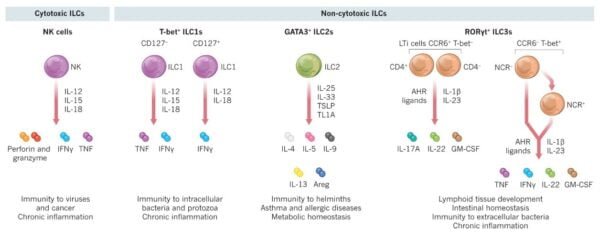
2. Key Functions of ILCs
- Cytokine Production: ILCs produce a range of cytokines, including interferon-gamma (IFN-γ), interleukin-5 (IL-5), and interleukin-13 (IL-13). These cytokines help regulate immune responses and inflammation. For instance, IFN-γ plays a critical role in enhancing the ability of other immune cells to combat infections.
- Antiviral Mechanisms: By producing antiviral cytokines and upregulating genes involved in antiviral defense, ILCs contribute to increased resistance of mucosal surfaces to viral infections. This function is crucial in maintaining mucosal immunity and preventing pathogen entry.
Summary
Innate immunity is the body’s initial and broad-spectrum defense mechanism against infections and injuries. It involves a range of components, including ILCs, NK cells, and various internal and external defense mechanisms. While innate immunity lacks the specificity of adaptive immunity, it plays a crucial role in providing immediate protection and setting the stage for the adaptive immune response.
By understanding the functions and interactions of innate immune cells and mechanisms, we gain insight into how the body defends itself against disease and how these processes can be utilized or modified in medical research and treatment strategies.
References
- Janeway, C. A., Travers, P., Walport, M., & Shlomchik, M. J. (2001). Immunobiology: The Immune System in Health and Disease. 5th Edition. Garland Science.
- Medzhitov, R., & Janeway, C. A. (2000). “Innate Immunity.” New England Journal of Medicine, 343(5), 338-344.
- Gordon, S. (2002). “Pattern Recognition Receptors: Doubling Up on Detection.” Nature Reviews Immunology, 2(5), 332-341.
- Klose, C. S. N., & Artis, D. (2020). “Innate Lymphoid Cells as Regulators of Immunity, Inflammation, and Tissue Homeostasis.” Nature Immunology, 21(5), 498-508.
- Murphy, K., & Weaver, C. (2016). Janeway’s Immunobiology. 9th Edition. Garland Science.
- Takeda, K., & Akira, S. (2004). “Toll-Like Receptors in Innate Immunity.” International Immunology, 16(1), 1-14.
- Bianchi, M. E. (2007). “DAMPs, PAMPs, and Alarmins: All We Need to Know About Danger.” Journal of Leukocyte Biology, 81(1), 1-5.