Author: Binod G C
Abstract
For the cell cycle to be accomplished and get exhibited intracellularly for cell growth, cells undergo some processes with extracellular matrix proteins (ECM) through integrins mediation to induce differentiation, motility and survival as a by-product of the integrins signaling pathway. Integrin activation signaling complex employ 3 state of conformation; 1) bent and clasped state which is inactive and has a low affinity to ECM binding 2) extended state with moderate high affinity which induce inside-outside signaling upon partial activation of Talin and Kindlin which expose binding motifs and 3) open extended state that induces outside-inside signaling upon ECM binding that stabilizes integrin and exhibit full interaction with other intracellular proteins/kinases. Integrins signaling activate RAS-PI3K/Akt pathway to induce cell growth/survival, RAS/MAPK to induce cell proliferation and Rac1/RhoA/cdc42 pathway for cell motility.
Therefore, this term paper will somehow elucidate on integrin signaling activation mechanism in the cells and its relations with other signaling pathways, to drive the cell cycle. It will also focus on the role of integrins pathway in the cell and extracellular-intracellular responses once the integrins receptors/ligand are non-functional or inhibited
Keywords: integrins signaling, ECM, integrins, cell cycle, cell migration
Background on Integrins
Integrins are heterodimeric transmembrane proteins with multiple alpha (α) and beta (β) subunits which correspond with typical extracellular matrix as ligands to activate signaling events (Campbell and Humphries 2011). Extracellular matrix proteins (Laminins, fibronectin, etc.) which are produced by fibroblast are used as integrins’ ligands.
Ligand-integrins interaction promotes bone formation, osteoblastic differentiation(Marie et al., 2014) and derives growth factor signaling pathways such as RTK, EFGR (Giancotti. FG and Ruoslahti. E, 1999; Wei et al., 2015). For bone formation, the ligand binds to integrins receptor to activate the Focal Adhesion Kinases (FAK) via Talin and Kindlin interaction which induces RAS/MAPK to activate the ERK1/2 that subsequently enters into the nucleus to interact with the transcription factor RUNX2, responsible for bone formation and osteoblast differentiation (Marie, Haÿ, and Saidak 2014). Laminin, fibronectin, collagen, and vitronectin are integrins interacting proteins in the ECM that possess an integrin-binding motif (Wei et al., 2015). In case of integrins binding to ligands, these ECM proteins form a meshwork by interacting with one another to withstand the topography and compressional forces exerted on cells and therefore prepare the cell for any mechanical or physiological impact.
Ligand-integrins interaction in concert with growth factor promote cells to grow and differentiate. Similarly, the Growth factor from the plasma membrane interacts with the receptor (EGFR, RTK) to induce the accumulation of integrins which triggers the integrin signaling pathway to promote RAS/MAPK and PI3K/Akt for proliferation, survival and differentiation(Wei et al., 2015). In case of tumor or cancer, the extracellular matrix proteins interactions with integrins receptors are displaced and deteriorate with cancer progression where either the ECM or integrins level in plasma membrane decline thus blocking binding efficiency and cells’ stability (Cooper J and Giancotti FG 2017). This term paper focuses on the canonical integrins signaling pathway and speculate on some cross-talk between other signaling pathways with integrins signaling.
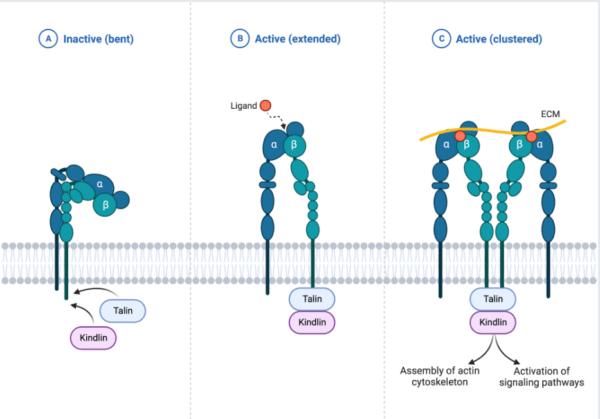
Extracellular Matrix proteins (ECM) and integrins signaling
ECM refers to proteins network-complex formed in the cell to provide cell anchorage and cell to cell communication that stimulates intracellular transport and signaling. The ECM proteins are classified into proteoglycans ECM that are made of glycosaminoglycans chains which strengthen connective fibrous tissues against compressional pressure, and Fibrous proteins ECM that provide structural support in the cell, to facilitate signal transduction such as cell division, migration, proliferation, and survival. The ECM proteins (fibrous protein) possess the integrin-binding motif that enhances interaction among them and associate with the integrins receptors to induce integrins signaling. Also, integrins receptors interact with the growth factor. They mostly involved fibrous protein ECM for integrins receptor interaction and cell adhesion include Laminin, Collagen, Fibronectin and Vitronectin that act as integrins receptor’s ligands to induce integrin signaling, in a different role within the cell (Wei et al. 2015)
Laminin
Laminin is an important extracellular matrix protein and atypical fibrous protein that is localized at the basal lamina of the epithelial surface of the cells (Campbel & Terranova, 1988). It is an important protein for embryo development and aid in attachment of epithelial cells into basal membrane thereby stimulating cell migration, differentiation, cell spread, invasion and survival (Campbel & Terranova, 1988; Sasaki et al, 2004). It is recruited into the basal membrane through binding to integrin receptors or transmembrane glycoprotein to influence cell cycle.
Collagen
Collagen is an important fibrous protein that forms connective tissues and provides structural support and cushion throughout the cell/body. Its classified into classes that have a specific binding motif to interact with the integrins receptor to enhance cell differentiation, migration, and survival. Collagen is very essential in embryogenesis since the mutation of collagen type I causes bone formation disruption leading to brittle bone disease due to lack of strong interaction with the integrins(Jokinen et al. 2004)
Fibronectin
Fibronectin is initially proposed to be localized in the blood where it is then secreted throughout the cell surface. It interacts with the integrin receptor at the plasma membrane to induce cell cycle through integrins signaling where it mediates cell-cell adhesion and migration through the formation of fibril(Akiyama et al, 1990).
Integrins signaling pathway cascade
Integrins are more often expressed in metazoan, sponges, and bilataria (Gahmberg C.G et al, 2009), and are described as an extracellular matrix receptor that binds to multiple ligands and characterized by heterodimeric transmembrane proteins and primarily binds to extracellular matrix proteins, it is made of two major subunits (alpha and beta) (Campbell and Humphries 2011). The integrins signaling plays a vital role in the cell cycle; proliferation, migration, differentiation, invasion, cell self-regeneration, and cell survival. Few of the major proteins and kinases that drive integrin signaling cascade include; Tallin, Kindlin Vinculin, FAK, Src Kinases, Paxillin and other integrin-linked kinases (ILK) (Harburger and Calderwood 2009; Cheah and Andrews 2018)
Tallin/Kindlin are major co-activators intracellular proteins that initiate the activation of integrins in the plasma membrane. The activation of Tallin and Kindlin break salt bridge that hold beta and alpha integrins closely together in inactive form to separate and open the exposure of binding site of divalent ion before ligands binding. Tallin interacts with the C-terminal tail of integrin(beta) through Kindlin protein association and majorly induce actin linkage (Cheah and Andrews, 2018) to stimulate stronger interaction between integrins and ECM proteins hence stabilize the cells from compressional effect throughout the cell cycle (Harburger and Calderwood 2009)
Vinculin mostly interacts with Tallin, Paxillin, and actin anchoring proteins such as F-actin, alpha-actinin and actin nucleating protein(ARP2/3) to mediate cell adhesion through associating with integrins thus facilitate cell migration and invasion (Cooper J and Giancotti FG 2017; Harburger and Calderwood 2009). Vinculin recruit more actin filaments to stabilize the matrix
Focal Activating Kinase (FAK) is a scaffold kinase protein-like in integrins complex that is the major protein and was the first to be discovered as a kinase that drives integrins signaling. FAK activation creates a binding site for the SH2 domain to dock that concomitantly phosphorylate it. It’s a ubiquitous protein in the cells and interacts with tallin, Kindlin and paxillin for the assembly of focal adhesion. It plays the role of cross-talk between growth factor signaling and Rac/RhoA pathway, and facilitate cell migration(Giancotti. FG and Ruoslahti. E 1999; Harburger and Calderwood 2009)
Src Kinase family is a major phosphorylation kinase protein with tyrosine kinase protein family (SFK) that phosphorylate FAK to induce focal adhesion by activating downstream kinase and Grb2/SOS adaptor proteins. After the assembly of focal adhesion, the FAK gets phosphorylated on serine to dissociate from Src and P130 during mitosis(Cooper J and Giancotti FG 2017; Harburger and Calderwood 2009). Moreover, the Src kinase family binds to the cytoplasmic tail of beta integrin to regulate cell migration.
Paxillin is another major scaffold protein that interacts with FAK and Integrin-like kinase through direct binding to alpha4 integrin tail to promote cell migration. It plays essential roles in protein to protein interaction through phosphorylation, cell adhesion turnover and regulates other multiple dynamic processes within the cell (Harburger and Calderwood 2009)
Integrin-Linked Kinases are also a major scaffold kinase protein that facilitates cell adhesion. It majorly forms a heterotrimeric complex with other kinases and paxillin binding proteins. It is connect to Ras/MAPK pathway and involved in the integrin activation through interacting with the Kindlin proteins(Harburger and Calderwood 2009).
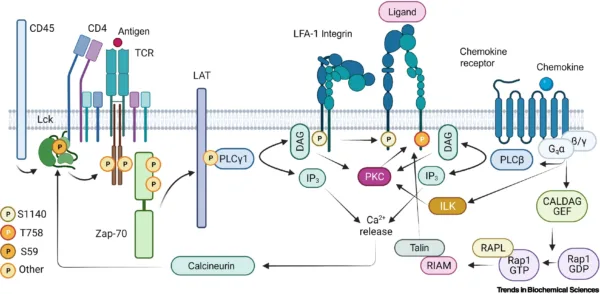
Source: https://doi.org/10.1016/j.tibs.2021.11.003
Integrins signaling cross-talk with other pathways
Integrins and other ECM proteins interaction provide cells anchorage that enable activation of tyrosine kinase in the plasma membrane to phosphorylate integrins subunits (beta3 and 4) which create conformational changes to associate with FAK for the recruitment of Src, shc, shp-2 and crk that serves as a link of crosstalk between RTK and integrins from canonical RAS-CRAF-MEK cascade phosphorylation (Soung et al., 2010).
On the other hand, the Src domain is activated which creates the feedback loop for mediating integrin signaling via phosphorylation of integrins and proteins that regulate Rho GTPases(Brunton et al., 2004). This shows that Src is the point of crosstalk between integrins signaling pathway and RTK where Src is activated by FAK or directly by integrins to recruit shc which induce nucleotide exchange (Grb2/sos1) to phosphorylate RAS-CRAF-MEK (Schlaepfer et al., 1998). However, in case of mutation of either Src or integrins, the cells lose fiber flexibility for ECM matrix proteins interaction, the ability of receptors to bind to ligand and reduction of focal adhesion protein activity that negatively triggers the phosphorylation cascade and protein interactions.
Integrins and growth factor receptor (GFR) do cross-talk to transcriptionally regulate the downstream effector. They both regulate P13k, MEK/ERK, and GTPases (both Rho and Rac) pathways. For instance, in the case EGFR protein levels is reduced, the integrins level is as well as reduced(Moreno-Layseca and Streuli 2014). This indicate the signal cross-talk between these two receptors that are activated from a different pathway.
Conclusion and Future perspective
Integrins signaling involves activation of integrins protein through interaction with an extracellular matrix for intracellular signals as well as extracellular signals that are exhibited from the cell. The ECM proteins (eg; laminin, fibronectin) bind to integrin receptors to recruit FAK through Kindlin and when FAK is activated, it creates a docking site for the Src domain for phosphorylation. The Src domain then activates the downstream effectors paxillin to recruit Talin that subsequently recruit actin linkage and Rac1/CDC42/RhoA pathway that induces lamellipodia/filopodia/stress fiber respectively for locomotion.
Upon growth factor, the FAK activate RAS-MAPK for proliferation and ILK activate PI3K/Akt pathway for cell survival/growth. For the integrins signal to take place, the epithelial cell is well anchored, supported and linked to the basal membrane (basal lamina) to prevent the activation of programmed cells and strengthen cell physiological mechanism. This show that, the integrin activation is a multi-processes step that take time for integrins to be switched from hooked state(low affinity conformation) to open extended state(high conformation) through intracellular proteins activation. Also some anchoring proteins recruit more actin cytoskeleton (Tallin, Paxillin, Vinculin) and bring firm association among the ECM proteins.
We have also seen that the cellular signaling pathways are directly or indirectly connected from downstream to drive downstream activation. However, much need to be done to clearly understand the cascade mechanism of the integrins signaling pathway. Much of genetic analysis in integrin signaling in embryo development, non-hematopoetic cells and disease-associated should be done to understand the mechanism behind and how the therapy on collagen type I can be done. Besides, sometimes the pathway is not clear on how it occurs and therefore the combination of many pathway and research need to be done to understand how this pathway influence cell growth, shape and migration and not the other pathways.
Reference:
- Akiyama SK., Nagata K, and Yamada KM, (1990). Cell Surface Receptors for Extracellular Matrix Components. BBA – Reviews on Biomembranes 1031 (1): 91–110. https://doi.org/10.1016/0304-4157(90)90004-V.
- Brunton V.G., MacPherson J, and Frame MC, (2004). Cell Adhesion Receptors, Tyrosine Kinases and Actin Modulators: A Complex Three-Way Circuitry. Biochimica et Biophysica Acta – Molecular Cell Research 1692 (2–3): 121–44. https://doi.org/10.1016/j.bbamcr.2004.04.010.
- Campbel J.H and Terranova V.P, (1988). Laminin: Molecular Organization and Biological Function. J Oral Pathol 17: 309–23. https://doi.org/10.1111/j.1600-0714.1988.tb01543.x.
- Campbell I.D., and Humphries MJ, (2011). Integrin Structure, Activation, and Interactions. Cold Spring Harbor Perspectives in Biology 3 (3): 1–14. https://doi.org/10.1101/cshperspect.a004994.
- Carl G. Gahmberg, Susanna C. Fagerholm, Susanna M. Nurmi, Triantafyllos Chavakis, Silvia Marchesan, and Mikaela Grönholm, (2009). Regulation of Integrin Activity and Signalling Silvia. Biochim Biophys Acta. 1790 (6): 431–44. https://doi.org/10.1016/j.bbagen.209.03.007.Regulation.
- Cheah M, and Andrews M. 2018. “Integrin Activation: Implications for Axon Regeneration.” Cells 7 (3): 20. https://doi.org/10.3390/cells7030020.
- Cooper J and Giancotti FG. 2017. “Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance.” Cancer Cell. 176 (5): 139–48. https://doi.org/10.1016/j.physbeh.2017.03.040.
- Giancotti. FG and Ruoslahti. E. 1999. “Integrin Signaling.” REVIEW: SIGNAL TRANSDUCTION 285 (5430): 1028–33. https://doi.org/DOI: 10.1126/science.285.5430.1028.
- Harburger, David S., and David A. Calderwood. 2009. “Erratum: Integrin Signalling at a Glance (Journal of Cell Science Vol. 122 (159-163)).” Journal of Cell Science 122 (9): 1472. https://doi.org/10.1242/jcs.052910.
References
- Jokinen, Johanna, Elina Dadu, Petri Nykvist, Jarmo Käpylä, Daniel J. White, Johanna Ivaska, Piia Vehviläinen, et al. 2004. “Integrin-Mediated Cell Adhesion to Type I Collagen Fibrils.” Journal of Biological Chemistry 279 (30): 31956–63. https://doi.org/10.1074/jbc.M401409200.
- Marie, Pierre J., Eric Haÿ, and Zuzana Saidak. 2014. “Integrin and Cadherin Signaling in Bone: Role and Potential Therapeutic Targets.” Trends in Endocrinology and Metabolism 25 (11): 567–75. https://doi.org/10.1016/j.tem.2014.06.009.
- Moreno-Layseca, Paulina, and Charles H. Streuli. 2014. “Signalling Pathways Linking Integrins with Cell Cycle Progression.” Matrix Biology 34: 144–53. https://doi.org/10.1016/j.matbio.2013.10.011.
- Sasaki, Takako, Reinhard Fässler, and Erhard Hohenester. 2004. “Laminin: The Crux of Basement Membrane Assembly.” Journal of Cell Biology 164 (7): 959–63. https://doi.org/10.1083/jcb.200401058.
- Schlaepfer, David D., and Tony Hunter. 1998. “Integrin Signalling and Tyrosine Phosphorylation: Just the FAKs?” Trends in Cell Biology 8 (4): 151–57. https://doi.org/10.1016/S0962-8924(97)01172-0.
- Soung, Young Hwa, John L. Clifford, and Jun Chung. 2010. “Crosstalk between Integrin and Receptor Tyrosine Kinase Signaling in Breast Carcinoma Progression.” BMB Reports 43 (5): 311–18. https://doi.org/10.5483/BMBRep.2010.43.5.311 .
- Wei, Qiang, Theresa L.M. Pohl, Anja Seckinger, Joachim P. Spatz, and Elisabetta A. Cavalcanti-Adam. 2015. “Regulation of Integrin and Growth Factor Signaling in Biomaterials for Osteodifferentiation.” Beilstein Journal of Organic Chemistry 11: 773–83. https://doi.org/10.3762/bjoc.11.87.