When a signal molecule binds to a receptor protein, it causes to change its shape (conformational /structural changes). The ligands (“first messengers”) can carry signals and, interact with other intracellular molecule, the second messengers. The intracellular signaling molecules termed as second messengers of certain low-molecular-weight which are a short-lived and can increase (or decrease) in the concentration that causes a change in the function of enzymes or non-enzymatic proteins. GPCR Signaling is described in detail in this article.
Some of the examples of secondary messengers are
- 3,5-cyclic AMP (cAMP),
- 3,5- cyclic GMP (cGMP),
- 1,2-diacylglycerol (DAG)
- Inositol 1,4,5-trisphosphate (IP3)
- Ca++ and various inositol phospholipids( phosphoinositides).
Signaling by G protein coupled receptor
The way the signals are transduce is different in various intracellular pathways that transduce signals downstream from activated cell-surface receptors. G protein coupled receptors indirectly activate enzymes that generate intracellular second messengers as mentioned above. They combine through GTP-binding proteins, or G proteins in order to do so. Example-adrenergic receptor system that detects epinephrine (adrenaline). Intracellular signaling with G protein coupled receptors (GPCR Signaling) comprises of following cascades described in the points below;
1.G-protein coupled receptor in GPCR Signaling
GPCRs are the largest and most diverse group of membrane receptors as human genome encodes about 800 different GPCRs. Diverse ligands responsible for different sensory receptors ranging from photons, ions, amino acids, odorants, bitter and sweet gustatory substances, pheromones, eicosanoids, neurotransmitters, peptides, proteins, and hormones are known to interact with GPCRs which eventually regulates wide variety of physiological functions.
Structure of GPCR
Though GPCRs has variety of signaling molecules, they share a common architecture which has been conserved during evolution. They are known as heptahelical receptors, serpentine receptors or seven transmembrane receptors due to seven-helix bundle of GPCRs which is flexible and capable of changing many conformations. According to the signals received or the ligands, they can stabilize distinct conformations and therefore transmit the signal to distinct members of the family of heterotrimeric G proteins.
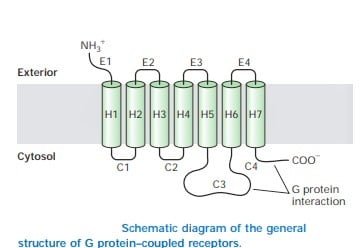
Source: Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th edition
Thus the interaction occurs functionally with membrane-spanning receptors and appropriate effectors.
2. GTPase Switch Proteins
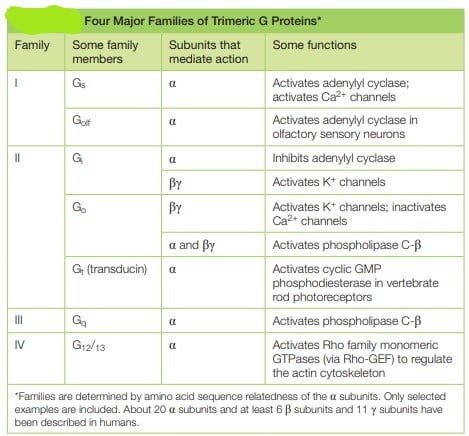
There are two classes of GTPase switch proteins:
- Trimeric (large) G proteins (receptors proteins)
- Monomeric (small) G proteins such as Ras and various Ras-like proteins.
The G proteins contain regions like switch I and switch II which control the activity of specific effector proteins by direct protein-protein interactions when the G protein is bound to GTP. Thus the ability of a G protein to interact with other proteins and the transduction of signal differs in the GTP-bound “on” state and GDP-bound “off” state.
(a) During the active “on” state, two domains, termed switch I (green) and switch II (blue), as shown in figure, the domains are bound to the terminal phosphate of GTP. This occurs through the interactions with the backbone amide groups of a conserved threonine and glycine residue.
(b) The release of the phosphate by GTPase-catalyzed hydrolysis causes switch I and switch II to relax into a different conformation, enabling the inactive “off” state.
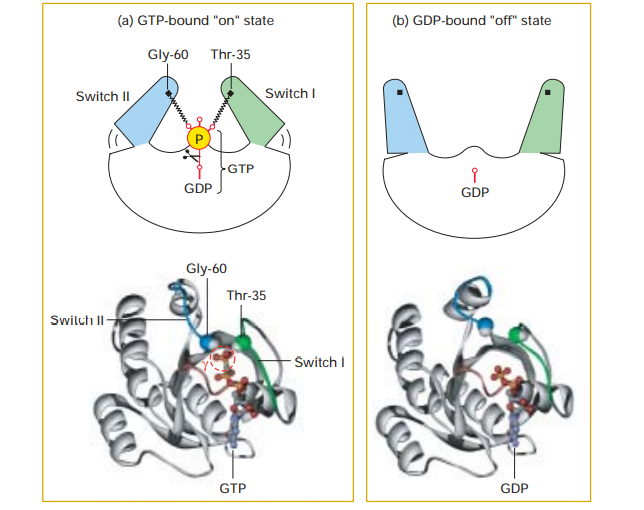
Source: Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th edition
Trimeric G Proteins Relay Signals from GPCRs in GPCR Signaling
They referred to as G proteins because they bind guanine nucleotides, either GDP or GTP. There are several families of trimer G-proteins. heterotrimeric G protein consists of three different polypeptide subunits, called α, β and γ held at the plasma membrane by lipid chains that are covalently attached to the α and γ subunits. Heterotrimeric GTP-binding proteins associate with membranes through hydrophobic tails and remain attached to C-termini of the alpha and gamma subunits.
- The guanine nucleotide-binding site is present on the Gα subunit. As mentioned in above mechanism, the binding of Gα subunit to GTP,depicts that they are ‘on’, leading to cascade of signal transduction and when they are bound to GDP, they are ‘off’ leading to termination of signals.
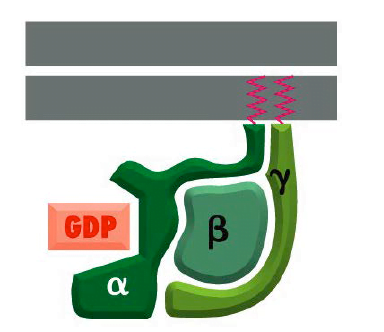
Structure of Trimeric G protein
Source;Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. 2002. Molecular Biology of the Cell.
3. Protein Kinases and Phosphatases in GPCR Signaling
The activation of all cell surface receptors eventually leads to changes in protein phosphorylation through the activation of protein kinases or protein phosphatases via secondary messengers. The human genome is capable of encoding 500 protein kinases and 100 different phosphatases. There are two types of protein kinases in animal cells: those that add phosphate to the hydroxyl group on tyrosine residues and those that add phosphate to the hydroxyl group on serine or threonine (or both) residues. However, phosphatases, which remove phosphate groups, can act in concert with kinases to switch the function of various proteins on or off.
The receptor itself possesses intrinsic kinase or phosphatase activity in some signaling pathways whereas in other pathways, the receptor interacts with cytosolic or membrane associated kinases.
4. GPCR signaling mechanism
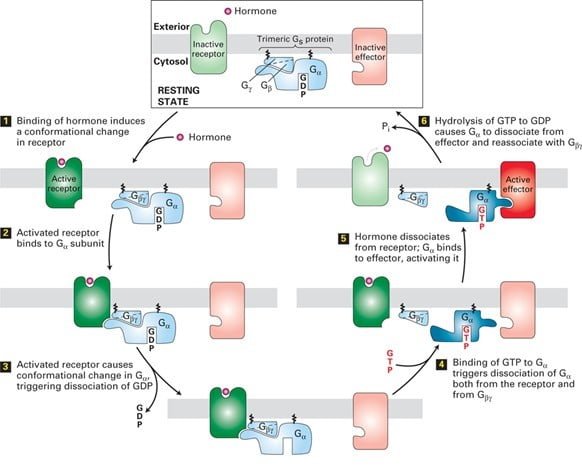
The signals are most commonly transduced by Gα, a GTPase switch protein that alternates between an active (“on”) state with bound GTP and inactive (“off”) state with GDP. The other ɤ and β subunits, which remain bound together, occasionally transduce signals in GPCR Signaling.
A typical example is the hormone-occupied receptors act as GEFs for G proteins.
The catalytic dissociation of GDP and enabling GTP to bind results in change in conformation of switch regions in G causes it to dissociate from the Gα subunit and interact with an effector protein with the binding of the ligand as shown in the figure. The hydrolysis of GTP terminates signaling and leads to reassembly of the trimeric form, returning the system to the resting state. Thus the repetition of the cycle occurs with the binding of another ligand molecule. In some of the pathways, the effector protein is activated by the free Gɤβ subunit.
Learn more about
References
- Iwasa, J., Marshall, W. F., & Karp, G. (2016). Karp’s cell and molecular biology: Concepts and experiments
- Krauss G (2008). Biochemistry of Signal Transduction and Regulation. Wiley-VCH. p. 15. ISBN 978-3527313976.
- Hu, G., Mai, T. & Chen, C. (2017). Visualizing the GPCR Network: Classification and Evolution. Sci Rep 7, 15495 https://doi.org/10.1038/s41598-017-15707
- Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. 2002. Molecular Biology of the Cell. New York: Garland Science. pp 830-850
- Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th edition. Pp 543-567
- https://www.alpfmedical.info/plasma-membrane/intracellular-signal-transduction.html
- https://www.slideshare.net/AliBarakat2/cell-signaling-43255324
- https://www.scribd.com/document/23277709/csir
- https://www.brainscape.com/flashcards/lecture-2-3942940/packs/5772346
- https://biodesign.asu.edu/news/research-opens-door-improved-drugs-type-ii-diabetes
- https://slideplayer.com/slide/8071882/
- http://tbl.med.yale.edu/cell_communication_2020/reading.php