What are Lipids?
Lipids are a large family of organic molecules that are hydrophobic but soluble in organic solvents such as alcohol and ether. They are an essential part of live cells and conduct a variety of biological tasks. Lipids are principally made of carbon and hydrogen atoms, with trace quantities of oxygen, nitrogen, and other elements. Lipid metabolism refers to the processes that occur in the body to produce, transport, store, and use lipids.
Lipids are classified as fats, oils, waxes, phospholipids, and steroids. Fats and oils are often utilized for energy storage in the body, whereas waxes are employed for waterproofing and protection. Phospholipids are the primary components of cell membranes, which serve as a barrier between the inside of cells and their external environment. Steroids, such as cholesterol, serve a variety of activities in the body, including functioning as precursors of hormones.

Lipid metabolism
Lipid metabolism refers to the processes that occur in the body to produce, transport, store, and use lipids. This comprises dietary lipid breakdown, lipid synthesis, and lipid conversion into other biomolecules.
The breakdown of dietary lipids occurs in the digestive tract, where lipases break down fats into fatty acids and glycerol. These components are subsequently taken into the circulation and distributed to various tissues, where they either provide energy or are stored as fat.
The liver and adipose tissue are the primary sites for lipid production. Triglycerides are formed by the combination of fatty acids and glycerol and are subsequently transferred to adipose tissue for storage or utilized for energy by other tissues.
Lipids can be transformed into other biomolecules as well. Cholesterol, for example, may be generated from fatty acids and utilized to make hormones, vitamin D, and bile acids. Fatty acids can also be utilized to make complex lipids like phospholipids and sphingolipids, which are essential components of cell membranes.
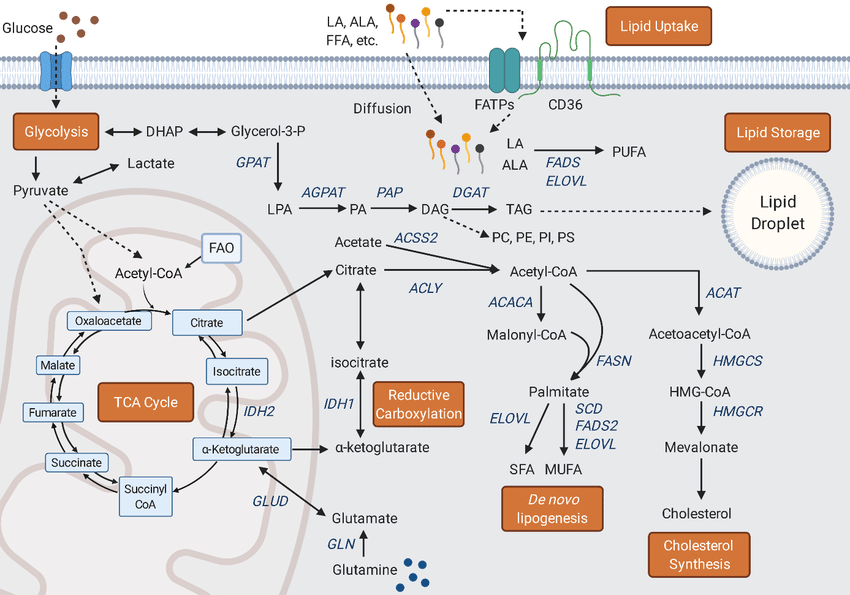
Types Lipid metabolism
Lipid metabolism is classified into two types: anabolic and catabolic.
Anabolic lipid metabolism is the process by which new lipids are synthesized from smaller molecules such as glucose and amino acids. This mechanism is critical for storing extra energy as triglycerides, which may be stored in adipose tissue for later use. Anabolic lipid metabolism takes place predominantly in the liver, where excess glucose and amino acids are transformed into fatty acids, which are subsequently turned into triglycerides.
Catabolic lipid metabolism is the breakdown of lipids into smaller molecules like fatty acids and glycerol, which may subsequently be utilized to produce energy. This process is crucial when the body has to break down stored fat for energy, such as while fasting or exercising. Catabolic lipid metabolism takes place predominantly in adipose tissue, where stored triglycerides are broken down into fatty acids and glycerol before being released into the circulation for utilization by other tissues.
Hormones such as insulin, glucagon, and adrenaline affect both anabolic and catabolic lipid metabolism. Insulin increases anabolic lipid metabolism by encouraging triglyceride synthesis, whereas glucagon and adrenaline stimulate catabolic lipid metabolism by promoting triglyceride breakdown. Overall, the balance of anabolic and catabolic lipid metabolism is critical for maintaining energy balance and avoiding metabolic diseases like as obesity and diabetes.
Lipolysis
Lipolysis is the breakdown of triglycerides (the body’s primary form of fat storage) into their constituent fatty acids and glycerol. When energy needs are high, this process happens largely in adipose tissue (body fat), although it can also occur in other tissues such as the liver and muscle.
Lipolysis is vital for delivering energy to the body during periods of fasting or low food consumption. It is also essential for maintaining a healthy body weight and preventing the development of obesity. Lipolysis dysregulation can result in a variety of health issues, including insulin resistance, diabetes, and cardiovascular disease.

Adrenaline (also known as epinephrine), glucagon, and cortisol are hormones that modulate lipolysis. These hormones promote lipolysis by attaching to adipocyte (fat cell) receptors and activating an enzyme known as hormone-sensitive lipase. This enzyme degrades triglycerides into fatty acids and glycerol, which are subsequently released into the circulation and used as a source of energy by other tissues.
Overall, lipolysis is a crucial process for giving energy to the body and controlling body weight. Hormones strictly control it, and it can be dysregulated in some metabolic diseases, resulting in a variety of health concerns.
Steps of beta oxidation
The process of breaking down fatty acids into acetyl-CoA molecules that may be utilized for energy generation is known as beta-oxidation. The following steps are involved in the process, which takes place in the mitochondria of cells:
- Activation: Fatty acids must be activated in the cytoplasm of cells before beta-oxidation may occur. This procedure includes the attachment of coenzyme A (CoA) to the fatty acid, resulting in the formation of a fatty acyl-CoA molecule.
- Transport: A transport protein situated in the inner mitochondrial membrane transports the fatty acyl-CoA molecule into the mitochondria.
- Oxidation: Once within the mitochondria, a series of oxidation events break down the fatty acyl-CoA molecule into acetyl-CoA molecules. In the first stage, two hydrogen atoms are removed from the fatty acid, resulting in the creation of a double bond between two carbon atoms.
- Hydration: The double bond is hydrated, or “wetted,” by the addition of a water molecule, resulting in the formation of a hydroxyl group (-OH) on one of the carbon atoms.
- Oxidation: An additional hydrogen atom is removed from the fatty acid, resulting in the creation of another double bond between two carbon atoms.
- Thiolysis: The fatty acid molecule is cleaved into a two-carbon acetyl-CoA molecule and a shorter fatty acyl-CoA molecule in the last step. The enzyme thiolase catalyzes the reaction, which releases energy in the form of ATP and reduced coenzymes (NADH and FADH2).

The acetyl-CoA molecule created by beta-oxidation can enter the citric acid cycle (also known as the Krebs cycle) to generate additional ATP via oxidative phosphorylation. Overall, beta-oxidation is a crucial mechanism for the breakdown of fatty acids and the creation of energy in the form of ATP.
Steps of ketogenesis
Ketogenesis is the process through which ketone bodies are synthesized in the liver from fatty acids. The following are the steps involved in ketogenesis:
- Fatty acid mobilization: Triglycerides held in adipose tissue are first broken down into free fatty acids and glycerol, which are then released into the circulation. After then, the free fatty acids are delivered to the liver.
- Fatty acid activation: Fatty acyl-CoA molecules are formed when free fatty acids bind to CoA. This process takes place in the cytoplasm of the liver cell.
- Beta-oxidation: The fatty acyl-CoA molecules are subsequently broken down into acetyl-CoA molecules by beta-oxidation. This process takes place in the mitochondria of the liver cell.
- Acetoacetate formation: A sequence of enzymatic processes converts the acetyl-CoA molecules generated by beta-oxidation into acetoacetate. This procedure is also carried out in the mitochondria of the liver cell.
- Formation of other ketone bodies: Acetoacetate can be transformed into two more ketone bodies, beta-hydroxybutyrate and acetone, via further enzyme processes.
- Ketone body release: These are Ketone bodies are released from the liver into the circulation, where they can be transferred to other tissues, such as muscle and brain, to be used as an energy source.
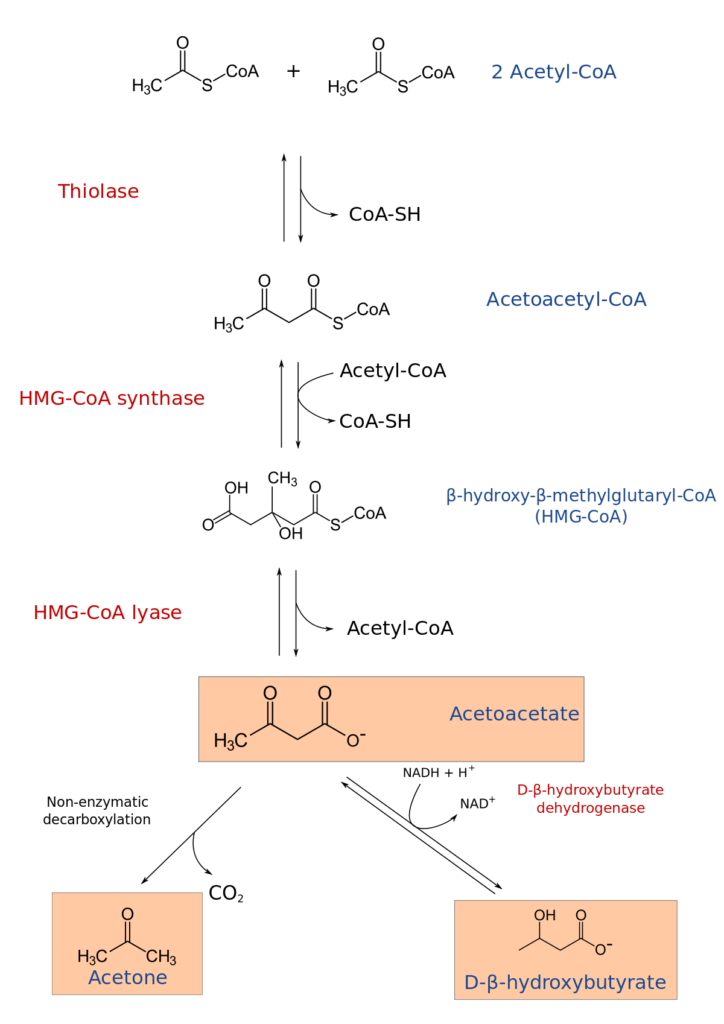
When the body’s glucose levels are low, such as during fasting or a low-carbohydrate diet, ketogenesis is often promoted. Ketone bodies, which are formed during ketogenesis, can serve as an alternate fuel source for the body, notably for the brain and other tissues that ordinarily rely on glucose for energy. Excessive ketone body synthesis, on the other hand, might result in ketoacidosis, a potentially deadly illness that happens when the blood gets excessively acidic.
Ketone body oxidation
The oxidation of ketone bodies is the mechanism through which the body utilizes ketone bodies (acetoacetate and beta-hydroxybutyrate) as an energy source. The following stages are involved in the oxidation of ketone bodies:
- Transport: Ketone bodies are carried from the liver, where they are created, to other tissues such as the brain, heart, and skeletal muscle. They circulate in the circulation and enter cells via particular transport proteins.
- Conversion to acetyl-CoA: Once within cells, ketone bodies are transformed to acetyl-CoA by a process known as ketolysis. The enzyme responsible for this reaction is known as succinyl-CoA:3-ketoacid CoA transferase (SCOT).
- Entry into the citric acid cycle: Acetyl-CoA generated by ketone body oxidation can enter the citric acid cycle, commonly known as the Krebs cycle, to create ATP via oxidative phosphorylation.
- ATP production: The oxidation of ketone molecules produces ATP in the mitochondria of cells via oxidative phosphorylation. This procedure comprises the movement of electrons down the electron transport chain, which results in the formation of a proton gradient across the mitochondrial membrane. The energy stored in this gradient is subsequently utilized to produce ATP via ATP synthase.

Overall, ketone body oxidation is a critical mechanism for supplying the body with an alternate source of energy during periods of low glucose availability, such as fasting or a low-carbohydrate diet. It helps the body to utilize stored fat as an energy source and can assist to avoid muscle loss during prolonged periods of food restriction.
Lipogenesis
Lipogenesis is the process through which the body transforms excess carbohydrates into triglycerides for storage in adipose tissue. Lipogenesis is comprised of the following steps:
- Glucose to acetyl-CoA conversion: Glucose is converted to acetyl-CoA by a sequence of processes known as glycolysis. This happens in the cell’s cytoplasm.
- Fatty acid synthesis: Acetyl-CoA is carried from the cytoplasm into the mitochondria and transformed into malonyl-CoA by the enzyme acetyl-CoA carboxylase. The enzyme fatty acid synthase then catalyzes a sequence of events that convert malonyl-CoA into fatty acids. This procedure takes place in the cell’s cytoplasm.
- Triglyceride formation: The fatty acids created in step 2 are subsequently mixed with glycerol to form triglycerides, the primary fat storage form in adipose tissue. The enzyme diacylglycerol acyltransferase (DGAT) catalyzes this reaction.
- Adipose tissue storage: The freshly produced triglycerides are packed into lipid droplets before being stored in adipose tissue for later use.
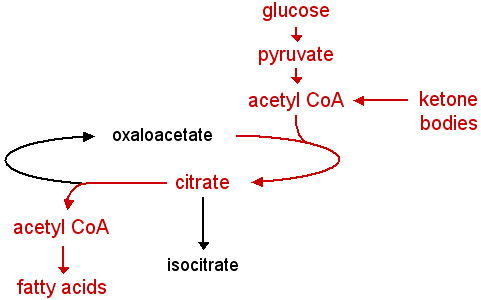
Lipogenesis is often increased by a high-carbohydrate, low-fat diet or by an increase in calorie consumption. Excess carbohydrates are transformed into fatty acids and stored in adipose tissue for later use. If calorie intake continually exceeds energy expenditure, this mechanism can lead to weight increase and the development of obesity.
Regulation of Lipid metabolism
Lipid metabolism is controlled by a complex network of pathways and feedback mechanisms that assist the body sustain lipid homeostasis. Here are some of the important regulators of lipid metabolism:
- Hormones: The hormones like insulin, glucagon, and leptin play an essential part in controlling lipid metabolism. Insulin enhances the absorption and storage of both fatty acids and glucose in adipose tissue, whereas glucagon accelerates glycogen breakdown and the release of glucose and fatty acids from the liver. Leptin regulates hunger and energy expenditure by acting on the hypothalamus.
- Transcription factors: Transcription factors are proteins that bind to DNA and control the expression of genes that regulate lipid metabolism. SREBP (sterol regulatory element-binding protein), PPAR (peroxisome proliferator-activated receptor), and LXR (liver X receptor) are three transcription factors involved in lipid metabolism.
- Enzymes: Enzymes play an important role in lipid metabolism by facilitating lipid production and degradation. Hormones, transcription factors, and other signaling pathways regulate enzymes involved in lipid metabolism.
- Dietary variables: Dietary factors such as the kind and amount of fat consumed can have an influence on lipid metabolism. Saturated and trans-fat-rich diets can raise LDL cholesterol and triglyceride levels, but unsaturated fat-rich diets can assist reduce these levels.
- Exercise: Regular exercise can improve lipid metabolism by improving fatty acid breakdown and use for energy generation.
Overall, lipid metabolism is a tightly controlled process that is influenced by a variety of physiological and environmental variables. This process’s dysregulation can result in a variety of metabolic diseases, including obesity, type 2 diabetes, and cardiovascular disease.
Significance of Lipid metabolism
Lipid metabolism is an essential mechanism for sustaining proper physiological function and health. Here are some of the important implications of lipid metabolism:
- Energy storage: The body may store surplus energy in the form of triglycerides, which can then be utilized as an energy source when needed. This is especially critical during times of lack of food or physical exercise.
- Energy generation: Lipid metabolism also helps the body to produce energy by breaking down stored fats in a process known as lipolysis. During periods of extended exertion or fasting, this mechanism is crucial.
- Structural function: Lipids are essential for the structure and function of cell membranes. Phospholipids, for example, make up the majority of cell membranes and are critical for their integrity and function.
- Hormone synthesis: Lipid metabolism is involved in the production of numerous essential hormones, including steroid hormones like testosterone and estrogen. These hormones have crucial roles in the control of several physiological processes, including growth, development, and reproduction.
- Brain function: Lipids are also necessary for normal brain function. The brain is around 60% fat, and lipids serve key roles in the building and sustaining of neuronal membranes, and in the transmission of nerve impulses.
Overall, lipid metabolism is essential for maintaining normal physiological function and health. Dysregulation of lipid metabolism can lead to a range of health problems, including obesity, diabetes, cardiovascular disease, and neurological disorders.
Lipid metabolism disorder
Lipid metabolism disorders are a set of medical illnesses caused by irregularities in the body’s fat processing and utilization. These diseases can be divided into two categories:
1. Lipid storage diseases: These illnesses are caused by an accumulation of lipids in numerous tissues and organs, which causes damage and malfunction. Gaucher disease, Niemann-Pick disease, and Tay-Sachs disease are examples of lipid storage diseases.
2. Lipid transport disorders: These illnesses are caused by anomalies in lipid transport throughout the body, resulting in excessive amounts of circulating lipids in the blood. Familial hypercholesterolemia, familial mixed hyperlipidemia, and hypertriglyceridemia are examples of lipid transport diseases.
Liver in lipid metabolism
The liver is essential for lipid metabolism. It has a role in lipid production, degradation, and transport throughout the body. Here are some of the liver’s important activities in lipid metabolism:
- Lipid synthesis: The liver is involved in the production of several essential lipids, such as cholesterol, phospholipids, and triglycerides. These lipids can be utilized to generate energy or stored in adipose tissue for future use.
- Lipid breakdown: The liver is also engaged in lipid breakdown via a process known as beta-oxidation. This mechanism transforms fatty acids into acetyl-CoA, which is subsequently utilized to generate energy.
- Lipid transport: The liver is in charge of lipoprotein production and secretion, which transports lipids throughout the body. Low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very-low-density lipoprotein (VLDL) are lipoproteins made up of lipids and proteins.
- Cholesterol regulation: The liver is important in the regulation of the body’s cholesterol levels. It produces cholesterol and removes excess cholesterol from the bloodstream via a mechanism known as reverse cholesterol transport.
- Bile production: Bile is produced and secreted by the liver, which aids in the emulsification of dietary fats in the small intestine and their absorption into the body.
In summary, the liver is important in lipid metabolism, and dysregulation of this process can result in a variety of health issues such as fatty liver disease, hyperlipidemia, and cardiovascular disease.