What are Macrolides?
Macrolides are a class of antibiotics that are commonly used to treat various bacterial infections. They belong to the larger group of antibiotics known as macrolide antibiotics, which are characterized by a large lactone ring structure with deoxy sugars attached.
The core structure of macrolides consists of a large lactone ring with multiple carbon atoms. This ring is usually composed of 14, 15, or 16 members, although some may have fewer or more members in the ring. The lactone ring is typically fused to one or more additional rings or substituents.

Attached to the lactone ring are various side chains and functional groups, which contribute to the specific properties and spectrum of activity of each macrolide. These side chains can differ in size, composition, and arrangement, resulting in structural variations among different macrolides.
For example, erythromycin, one of the first discovered and most well-known macrolides, has a 14-membered lactone ring and a cladinose sugar attached to one side of the ring. Azithromycin, a newer macrolide, has a 15-membered lactone ring and an additional methyl-substituted nitrogen in its structure.
The side chains and functional groups present in macrolides play a role in determining their pharmacokinetic properties, stability, and interactions with the bacterial ribosome.
Mechanism of Action of Macrolides
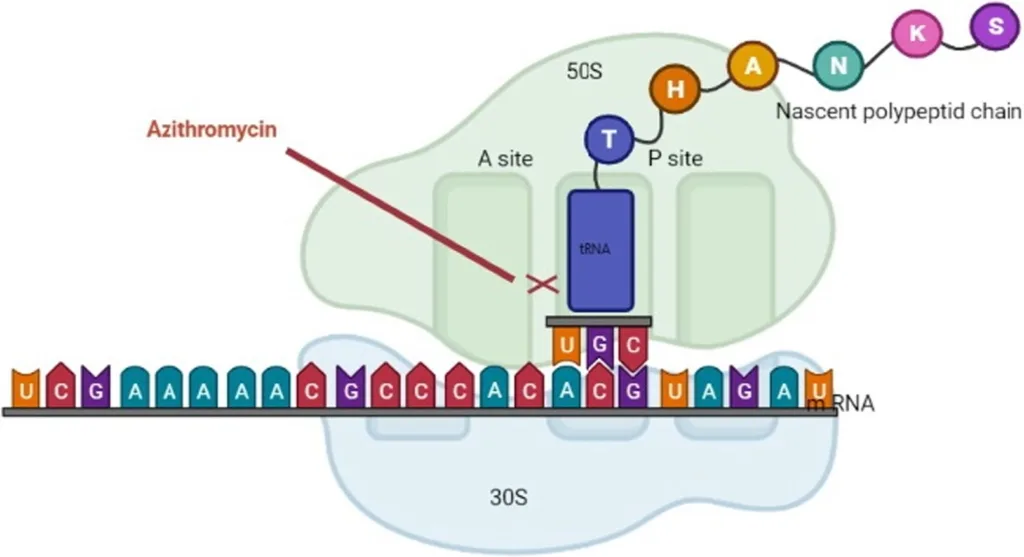
The mechanism of action of macrocodes has been extensively studied for over 30 years, although some aspects are still not fully understood.
- These antibiotics inhibit bacterial protein synthesis to varying degrees, with their specific target being the 50s ribosomal subunit and various ribosomal proteins, binding to the 23s ribosomal RNA molecule.
- Earlier studies suggest that 14-membered macrolides block the translocation of pepti-dyl-tRNA, while 16-membered compounds inhibit the peptidyl transfer reaction.
- The most recent hypothesis proposes that all macrolides stimulate the dissociation of peptidyl-tRNA from the ribosomes during the elongation phase, resulting in the inhibition of protein synthesis.
- They bind to the bacterial 50s ribosomal subunit, preventing the translation of mRNA and the addition of subsequent amino acids to the growing peptide chain by the enzyme peptidyltransferase.
- These are considered broad-spectrum as the bacterial ribosomal structure is highly conserved across most, if not all, bacterial species.
- Macrolides exhibit bacteriostatic effects by inhibiting protein synthesis, although they can be bactericidal at high doses.
- The anti-inflammatory and immunomodulatory effects of these antibiotics, especially azithromycin, are attributed to interactions with phospholipids and transcription factors like AP-1 and NF-kappaB, as well as other inflammatory cytokines.
- They interact with macrophages, leading to changes such as inhibition of cell function, cellular transport, and regulation of surface receptor expression, contributing to their immunomodulatory effects.
- Overprescription of antibiotics has resulted in significant resistance to many commonly used therapies, including macrolides. Bacterial resistance to macrolides primarily occurs through post-transcriptional methylation of the bacterial 23s ribosomal RNA.
- Resistance can be acquired through plasmid-mediated or chromosomal mechanisms, often facilitated by genetic mutations and the spread of resistance genes via transposable elements.
- Macrolides interfere with the formation of the polypeptide chain, mRNA translation, and protein synthesis, ultimately preventing bacterial growth.
- Inside bacterial cells, DNA forms a circular double strand containing the genetic code for essential proteins. Transcription results in the formation of mRNA, which attaches to the ribosome for protein synthesis.
- Bacterial ribosomes consist of 50s and 30s subunits that align tRNA molecules along the mRNA sequence to add amino acids and form a polypeptide chain through translation.
- The ribosome continues adding amino acids until it reaches a stop signal on the mRNA, releasing the finished protein molecule.
The exact mechanism of action is still being investigated, but they effectively inhibit bacterial growth by interfering with protein synthesis.
Antimicrobial Activity of Macrolides
The antimicrobial activity of macrolides is characterized by their ability to effectively combat various types of microbial infections. Here is an overview of the antimicrobial activity of macrolides:
- Broad-spectrum effectiveness: Macrolides exhibit broad-spectrum antimicrobial activity, meaning they are effective against a wide range of bacteria, including Gram-positive and some Gram-negative bacteria.
- Inhibition of protein synthesis: The primary mechanism underlying the antimicrobial activity of macrolides is their ability to inhibit bacterial protein synthesis. By binding to the 50s ribosomal subunit of bacteria, macrolides interfere with the formation of peptide bonds, preventing the synthesis of essential proteins needed for bacterial survival and growth.
- Bacteriostatic effects: Macrolides primarily exert a bacteriostatic effect, meaning they inhibit the growth and reproduction of bacteria rather than killing them outright. This allows the immune system to better control and eliminate the bacterial infection.
- Bactericidal effects at high concentrations: In certain situations, such as high drug concentrations or prolonged exposure, can also exhibit bactericidal effects. This means they directly kill bacteria rather than just inhibiting their growth.
- Effective against respiratory infections: Macrolides are commonly used to treat respiratory tract infections, including pneumonia, bronchitis, and sinusitis. They have shown efficacy against common respiratory pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae.
- Activity against atypical pathogens: Macrolides are particularly effective against atypical bacteria, such as Chlamydia pneumoniae and Legionella pneumophila, which are responsible for respiratory infections. They are also effective against other atypical organisms like Mycoplasma species.
- Additional antimicrobial indications: Macrolides have been utilized in the treatment of other infections, including skin and soft tissue infections, sexually transmitted infections (such as Chlamydia trachomatis), and certain gastrointestinal infections caused by Helicobacter pylori.
- Immunomodulatory effects: Macrolides possess immunomodulatory properties that extend beyond their direct antimicrobial activity. They can modulate the immune response, reducing inflammation and enhancing the body’s ability to fight infections.
- Combination therapy: Macrolides are sometimes used in combination with other antibiotics to enhance their efficacy or provide broader coverage against resistant bacterial strains.
- Resistance concerns: Like many antibiotics, the emergence of bacterial resistance to macrolides is a growing concern. This highlights the importance of judicious use and proper adherence to prescribed treatment regimens to minimize the development of resistance.
In summary, macrolides exhibit broad-spectrum antimicrobial activity, primarily by inhibiting bacterial protein synthesis. They are effective against various respiratory, atypical, and other types of bacterial infections. Macrolides also possess immunomodulatory effects and can be used in combination therapy. However, the emergence of resistance underscores the need for responsible antibiotic use.