Folding and assembly of multi-protein complex co-translationally or post-translationally is facilitated by the action of specialized proteins i.e. molecular chaperons. During the synthesis of polypeptides, some of the fraction of polypeptides released are in the native conformation therefore, a single chaperon acts on multiple client proteins
Molecular chaperones can be defined as the specialized proteins that interact with improperly folded polypeptides or partially folded, which corrects folding pathways or providing microenvironments for stabilization of folding intermediates and prevention of protein misfolding and aggregation.
Classes of Molecular chaperones:
There are different group of molecular chaperones, including Hsp60s, Hsp40, Hsp90s and sHsps, and Hsp70 proteins. Two major class are described below;
1.The first class, a family of proteins called Hsp70.
Hsp70/ Heat shock proteins
These proteins are more abundant in cells stressed by elevated temperatures such that named as heat shock protein. The hsp70 machinery acts early in the life of many proteins (often before the protein leaves the ribosome. They have indirect action of binding and stabilizing the unfolded or partly folded proteins that preventing these proteins from aggregation and degradation. . They also block the folding of some proteins that remains unfolded until they have been translocated across membranes. Some chaperones also facilitate the quaternary assembly of oligomeric proteins.
The heat shock proteins bind and release polypeptides in a cycle involving several other proteins (including a class called Hsp40) and ATP hydrolysis. They share an affinity for the exposed hydrophobic patches on incompletely folded proteins. These proteins recognize a small stretch of hydrophobic amino acids on a protein’s surface. Aided by a set of smaller hsp40 proteins, ATP-bound hsp70 molecules grasp their target protein and then hydrolyze ATP to ADP, undergoing conformational changes. This cause the hsp70 molecules to bind more tightly with the target. After the hsp40 dissociates, the rapid rebinding of ATP thus, induces the dissociation of the hsp70 protein after ADP release. Repeated cycles of hsp binding and release help the target protein to refold.
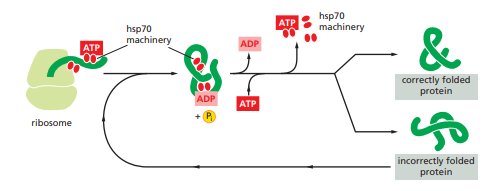
Figure 1: The hsp70 family of molecular chaperones.
[Source; Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Molecular Biology of the Cell. New York: Garland Science]
2.The second class of chaperones is called Chaperonins (Hsp60)
Chaperonins/Hsp60
Chaperonins, which directly facilitate the folding of proteins. They “protect” proteins that have been denatured by heat and peptides that are being synthesized (and are not yet folded).They also help to elaborate protein complexes that are required for the folding of a number of cellular proteins which do not fold appropriately.
About 10% to 15% of cellular proteins are known to require the chaperonin system, called GroEL/GroES, for folding under normal conditions (up to 30% require this assistance when the cells are heat stressed) in E. coli. They were first known to be necessary for the growth of certain bacterial viruses (hence the designation “Gro”). Unfolded proteins are bound within pockets in the GroEL complex, and the pockets are capped transiently by the GroES “lid”. GroES (a co-chaperonin, or hsp10) is a seven-subunit ring that sits on top of GroEL. GroEL (a chaperonin, or hsp60) is composed of two stacked, seven-subunit rings with a cavity in which ATP-dependent protein folding takes place.
GroEL undergoes s conformational changes, coupled to ATP hydrolysis. This causes the binding and release of GroES, thereby promoting folding of the bound polypeptide. Though structure of the GroEL/GroES chaperonin is discovered, many researches and function are yet to be known. Finally, the folding pathways of a number of proteins require two enzymes that catalyze isomerization reactions.
Protein disulfide isomerase (PDI)
that catalyzes the interchange or shuffling of disulfide bonds for the stable conformation. They also catalyze the removal of folding intermediates with inappropriate disulfide cross-links.
Peptideprolyl cis-trans isomerase (PPI)
catalyzes the interconversion of the cis and trans isomers of Pro peptide bonds, which can be a slow step in the folding of proteins that contain some Pro residue peptide bonds in the cis conformation.

Figure 2:-Space-Filling Model of the E. coli Chaperonin called the GroESGroEL Complex
[Source ;Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry].
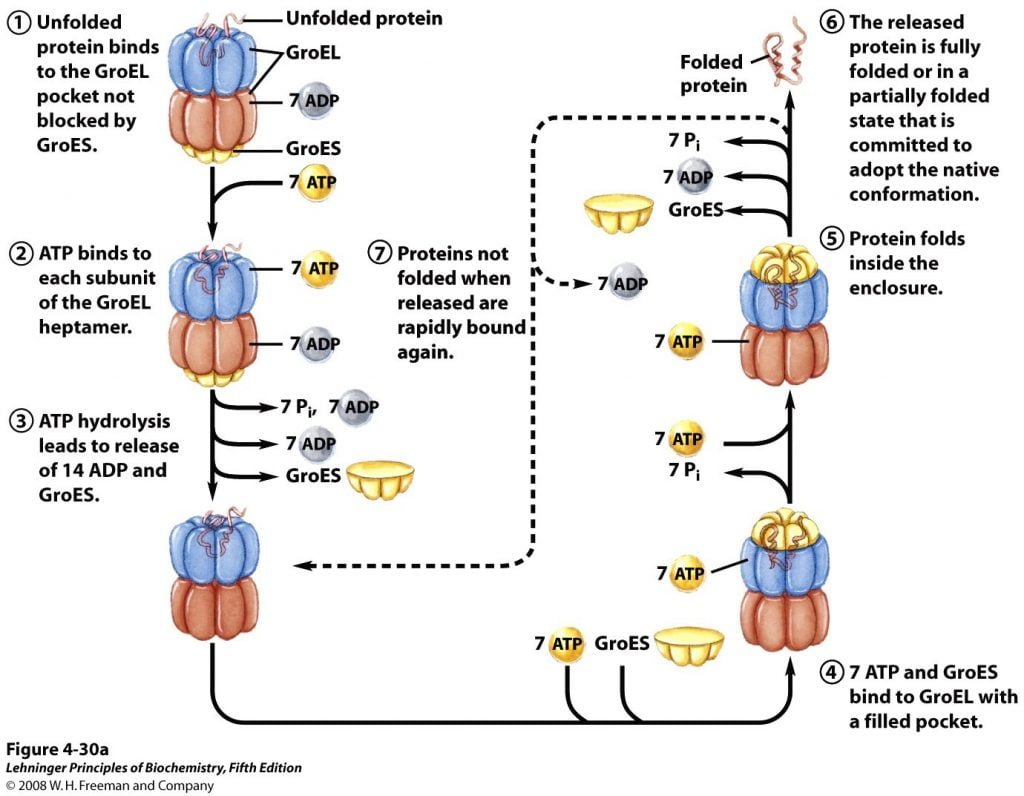
Figure 3; Illustration of Chaperonins Facilitate Folding
[Source ;Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry.]
Roles of chaperons
- “Chaperones,” are required for the proper folding of many species of proteins. Without chaperones, some of these pathways would not lead to the correctly folded in a stable form because the protein would become “kinetically trapped” in structures that are of-pathway. Some of these of-pathway configurations would aggregate and be left as irreversible dead ends of nonfunctional and potentially dangerous structures.
- Cells utilize several types of chaperones such as hsp as they are synthesized. As they reflects the operation of a feedback system that responds to an increase in misfolded proteins therefore it boosts the synthesis of the chaperones that help these proteins refold.
- Different members of these families including the hsp60 and hsp70 proteins function in different organelles. Mitochondria contain their own hsp60 and hsp70 molecules that are distinct from those that function in the cytosol; and a special hsp70 (called BIP) helps to fold proteins in the endoplasmic reticulum.
- Some of the chaperones are important in keeping the protein unfolded until its synthesis is finished. They act as catalysts by increasing the rates of the final stages in the folding process. Others protect protein fold so that their vulnerable, exposed regions do not become tangled in unproductive interactions.
References
- Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry. New York : W.H. Freeman, 2008 pp151-154
- Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Molecular Biology of the Cell. New York: Garland Science, 2002.pp 354-356
- Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th edition. Pp 69-70
- .https://www.nature.com/subjects/chaperones
- https://www.sciencedirect.com/topics/medicine-and-dentistry/chaperone
- https://pdb101.rcsb.org/motm/32
- https://reasonandscience.catsboard.com/t2590p25-origins-what-cause-explains-best-our-existence-and-why
- https://biomedpharmajournal.org/vol4no1/chaperone-co-chaperone-interactions-in-malarial-infection