Define Nitrogen cycle.
The nitrogen cycle is the biogeochemical process by which nitrogen in the environment gets converted into different chemical forms that living organisms may utilize. This cycle, which comprises a sequence of biological, chemical, and physical transformations in soil, water, and air, is critical for the survival of the ecosystems on Earth. Understanding and managing the nitrogen cycle correctly is critical for sustainable agriculture and conservation of the environment.
Steps of Nitrogen cycle
The nitrogen cycle consists of the following steps:
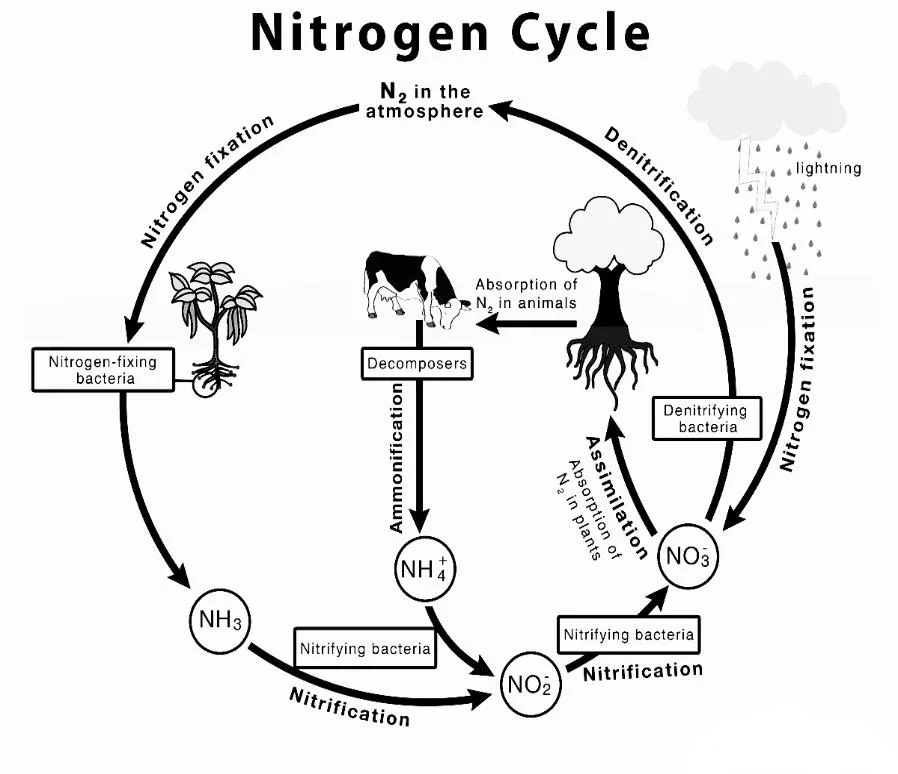
Step 1
Nitrogen Fixation
Nitrogen gas (N2) from the earth’s atmosphere is turned to ammonia (NH3) or ammonium (NH4+) by nitrogen-fixing bacteria present in soil, water, and the roots of some plants.
N2 + 8H+ + 8e– + energy → 2NH3
This process is carried out by nitrogen-fixing bacteria, which use energy from diverse sources such as sunlight or chemical compounds to turn atmospheric nitrogen gas (N2) into ammonia (NH3) or ammonium ions (NH4+).
Nitrogen fixing bacteria
Rhizobium, Bradyrhizobium, Azotobacter, Frankia, Cyanobacteria
Step 2
Nitrification
Nitrifying bacteria transform ammonia to nitrite (NO2) and finally into nitrate (NO3–). The steps of this process it takes take place in soil or water and requires oxygen.
NH3 + 2O2 → NO2– + 3H+ + 2e– NO2– + H2O → NO3– + 2H+ + 2e–
These processes are carried out out by nitrifying bacteria, that utilize oxygen to convert ammonia into nitrite (NO2–) and subsequently into nitrate (NO3–), releasing electrons and protons in the process.
Nitrifying bacteria
- Nitrosomonas (converts ammonia to nitrite)
- Nitrobacter (converts nitrite to nitrate)
Step 3
Assimilation
Plants uptake nitrate, which is utilized to synthesise nucleic acids and proteins.
- NO3– + H+ + e– → NO2– + H2O
- NO2– + H+ + e– → NH3 + H2O
Plants and other organisms carry out these processes by using nitrate or nitrite to produce amino acids and various nitrogen-containing compounds.
Step 4
Ammonification
Organic matter (dead plants and animals) is broken down by decomposers like bacteria and fungus, which releases ammonia back into the soil.
Organic nitrogen compounds → NH3
Decomposers, including bacteria and fungus, carry out this activity by dissolving the organic nitrogen molecules in dead plants and animals and releasing the ammonia back into the soil.
Ammonifying bacteria
Bacillus, Clostridium, Actinomycetes, Proteus, Pseudomonas, Escherichia coli
Step 5
Denitrification
Nitrate is reprocessed by certain bacteria into nitrogen gas, which is then discharged into the atmosphere. This process takes place in habitats with low oxygen levels, such as wet soils or silt at the bottom of lakes and rivers.
NO3– → NO2– → NO → N2O → N2
Denitrifying bacteria
Pseudomonas, Paracoccus, Alcaligenes, Bacillus, Achromobacter
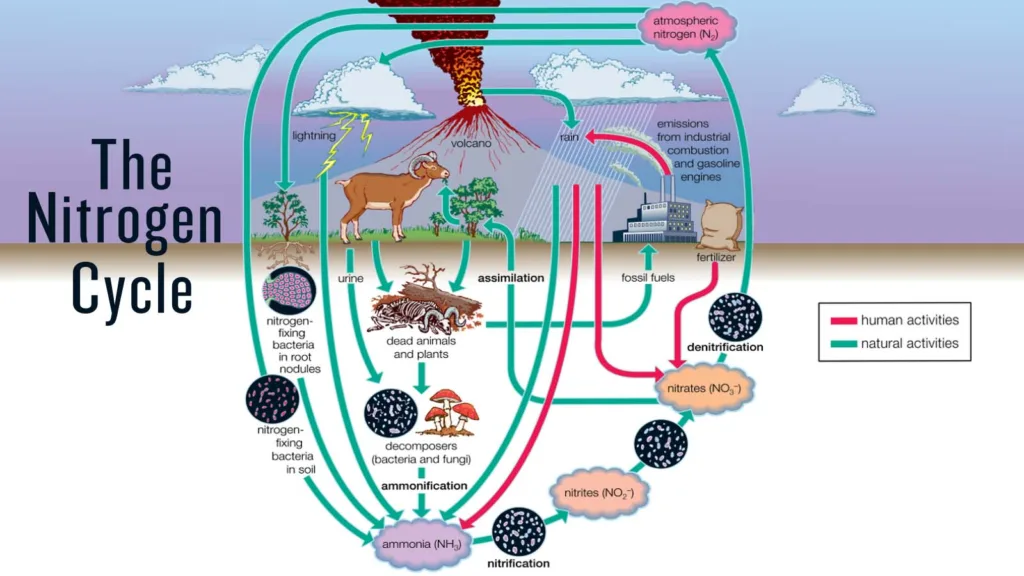
In natural ecosystems, the various stages are interrelated and continuously take place. The nitrogen cycle may go out of balance due to human activity, including the usage of fertilizers and the combustion of fossil fuels, which can have detrimental effects on the ecosystem.
Importance of Nitrogen cycle
The earth’s ecosystems cannot function and cannot survive without the nitrogen cycle. The nitrogen cycle is crucial for a number of reasons, some of which are listed below:
- Essential nutrient: The nitrogen cycle enables the conversion of nitrogen into a form that plants can use. Nitrogen is a necessary ingredient for plant development.
- Protein synthesis: Proteins and nucleic acids, which are necessary for the growth and reproduction of all living things, are made up mostly of nitrogen.
- Soil fertility: By replacing nutrients that plants deplete, the nitrogen cycle helps to maintain soil fertility.
- Environmental equilibrium: The nitrogen cycle contributes to the preservation of the nutrient balance in aquatic environments, limiting the accumulation of surplus nutrients that can result in algal blooms and other negative impacts.
- Climate control: By managing the quantity of nitrogen in the atmosphere, which can impact the amount of greenhouse gases there, the nitrogen cycle contributes to the regulation of the global climate.
What is Nitrogen fixation?
Nitrogen fixation is the process by which atmospheric nitrogen gas (N2) is converted into ammonia (NH3) or ammonium (NH4+) by certain microorganisms, such as bacteria or cyanobacteria. Nitrogen fixation is essential for life on earth, as nitrogen is an essential nutrient for plant growth, and plants form the base of the food chain.
Most plants and animals cannot use atmospheric nitrogen gas directly, as the nitrogen molecule is very stable and requires a lot of energy to break apart. Nitrogen fixation is therefore a crucial step in the nitrogen cycle, as it provides a source of usable nitrogen for plants and other organisms.
There are two main types of nitrogen fixation: biological and industrial.
- Biological nitrogen fixation occurs naturally in soil and water, where certain bacteria form symbiotic relationships with plants and convert atmospheric nitrogen gas into ammonia, which can be taken up by the plants.
- Industrial nitrogen fixation, on the other hand, involves the use of high-pressure and high-temperature conditions to convert atmospheric nitrogen into ammonia, which is used to produce fertilizers and other industrial chemicals.
Overall, nitrogen fixation is a key process in the global nitrogen cycle and plays a critical role in maintaining the health and productivity of ecosystems on earth.
What is Nitrogen fixing bacteria?
Nitrogen-fixing bacteria are a group of bacteria that have the unique ability to convert atmospheric nitrogen gas (N2) into ammonia (NH3) or ammonium (NH4+), which can be used by plants to synthesize amino acids and other nitrogen-containing compounds. These bacteria are commonly found in soil, water, and the roots of certain plants.
There are two main types of nitrogen-fixing bacteria:
Free-living nitrogen-fixing bacteria:
These bacteria are found in soil and water and can fix atmospheric nitrogen on their own without forming any symbiotic relationships with plants.
Some examples of free-living nitrogen-fixing bacteria include Azotobacter and Clostridium.
Symbiotic nitrogen-fixing bacteria:
These bacteria form symbiotic relationships with plants, where they live inside nodules on the roots of leguminous plants such as peas, beans, and clover. The bacteria fix atmospheric nitrogen and provide it to the plant, while the plant provides the bacteria with nutrients and a protective environment.
Examples of symbiotic nitrogen-fixing bacteria include Rhizobium and Bradyrhizobium.