For a full-grown organism to develop from a cell, the cell must divide. During the cellular division, a cell duplicates its contents and divides it into two. The main aim of the cell division is to accurately duplicate the organism’s DNA and its contents evenly. The cell cycle is completed in four phases with vigorous regulation at each steps. The cell division is regulated at different stages by various mechanisms at cell cycle checkpoints.
In eukaryotes, the cell cycle includes four phases:
- G1: cells actively grow and prepare for DNA replication.
- S phase: DNA replicates.
- G2: cells still grow and synthesize proteins required for division.
- M (mitosis) phase: duplicated chromosomes (sister chromatids) and cell contents divide into two daughter cells, each with a full copy of DNA.
The cell division process cannot be random and needs to be highly controlled as uncontrolled division might give rise to diseased states such as cancer. The monitoring and maintenance of genomic integrity during cell cycle occurs through a complex network of DNA repair pathways and cell-cycle checkpoints. The cell cycle checkpoints play a role in the system as they detect DNA damages and, in the repose, induce cell cycle arrest until the damage gets repaired. The mechanism of action of the cell cycle checkpoints is through the regulation of activities of cyclins and CDKs.
Cyclins and CDKs
Cyclin is the regulatory subunit because its concentration changes as cells progress through the cell cycle. The catalytic subunit is called CDKs and transfer phosphate groups from ATP to a particular amino acid residue within the substrates, phosphorylating them. CDKs show kinase activity only when associated with cyclin. Phosphorylation of proteins involved in cell-cycle control by cyclin-CDK complexes at their regulatory sites, either activates or inhibits them.

There are many checkpoints that regulates the cell cycle; however, the three major ones are
1. G1/S (restriction) checkpoint
Damage to DNA, availability of nutrients, and the growth factors are evaluated at the G1 checkpoint. If conditions are inadequate, the cell will no longer be allowed to enter into the S-phase. Stimulation of G0 cells with growth factors and mitogens induces expression of cyclin D, CDK4/6, and E2F transcription factor.
Role of p53 in DNA damage regulation
If there is significant DNA damage, p53 gets phosphorylated by kinases (ATM/ATR and Chk2/Chk1 kinases play a pivotal role), preventing it from binding with Mdm2. Mdm2 is a ubiquitin ligase that inhibits p53 by targeting it for degradation1. The p53 then stimulates the production of p21, a protein that binds and inhibits cyclin-CDK complexes, leading to cell cycle arrest until the DNA damage gets repaired and p21 level drops 2. If the DNA damage is irreversible, p53 triggers apoptosis (programmed cell death), preventing the duplication of damaged chromosomes.
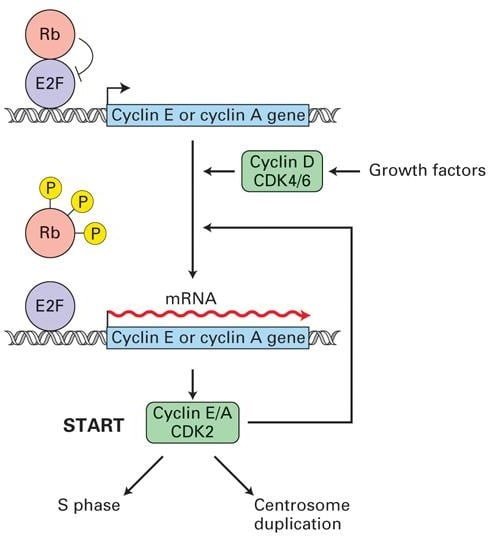
Role of Rb protein
Rb (tumor suppressor) protein binds to and inhibits E2F expression3. When the expression of the G1 cyclin-CDKs turns on, produced cyclinD-CDK4/6 complexes phosphorylate Rb protein, inactivating it and releasing the E2F transcription factor. E2F then stimulates expression of transcription genes required for entry into S-phase, especially genes encoding DNA synthesis enzymes, and S- phase cyclin-CDKs.
S-phase cyclinE/A-CDK2 complex initially remains inhibited until the G1/S phase cyclin-CDKs phosphorylate the inhibitors. After releasing, cyclinE/A-CDK2 phosphorylates regulatory proteins bound to chromosomal replication origins, promoting the initiation of DNA synthesis.
2. G2 checkpoint
The G2 checkpoint ensures the chromosomes are replicated and the replicated DNA of the cell is not damaged before entering mitosis. Mitotic cyclin B accumulates gradually and binds to CDK1. When cyclin B binds to CDK1, the resulting complex is known as MPF4. This complex acts as the signal for the G2 cell to enter mitosis and induce:
- Condensation of chromosomes,
- Nuclear envelope breakdown,
- Assembly of the mitotic spindle, and
- Alignment of condensed chromosomes at the equatorial region.
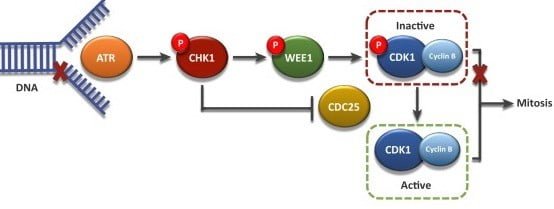
Before entry into mitosis, CDK1 is maintained in an inactive (phosphorylated) state by the kinase Wee1. During the G2/M transition, Wee1 gets phosphorylated by PLK1 5. Phosphorylated Wee1 is then degraded through the SCF ubiquitin ligase pathway. PLK1 additionally activates Cdc25 via phosphorylation. Tyrosine phosphatase cdc25 is responsible for dephosphorylation and activation of CDK1 6. The compound effect of Wee1 degradation and Cdc25 activation results in the net removal of an inhibitory phosphate group, activating cyclinB-CDK1 complex.
3. M checkpoint (spindle checkpoint)
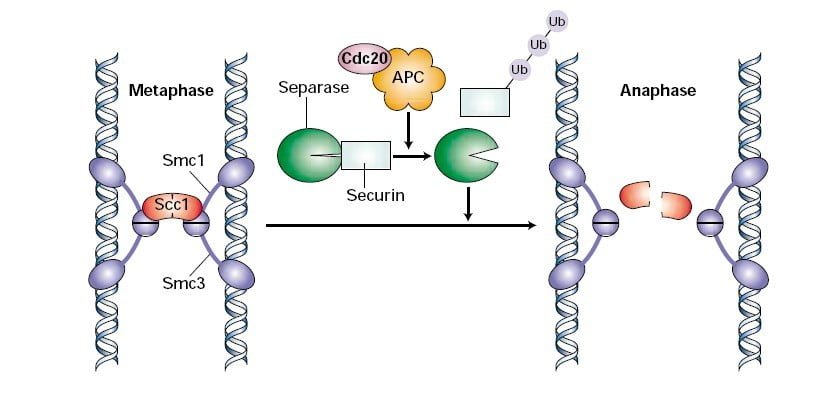
The M checkpoint is also known as the spindle checkpoint, as its primary role is to make sure all the sister chromatids are correctly attached to the spindle microtubules. Sister chromatids formed by DNA replication in the S-phase are held together at centromeres via ring-like proteins called cohesin complex. Cdc20 activates the APC/C ubiquitin ligase which is responsible for the polyubiquitination of securin. Securin is an inhibitor of the enzyme referred to as separase. Once proteasomes degrade securin, separase cleaves the Scc1 component of the cohesin complex, separating the sister chromatids7.
If there is an uneven tension and the microtubules are not properly attached to the chromosomes, this generates an error signal inside the cell. Mad2 detects the error signal and inhibits the activity of Cdc20. It delays the degradation of securin and metaphase to anaphase transition of the cell. The proper attachment of the spindle inactivates Mad2, liberating Cdc20 to trigger securin degradation.
Later during anaphase, APC/C polyubiquitinates mitotic cyclins leading to their degradation3. Decrease in mitotic cyclin-CDK kinase concentration leads to dephosphorylation of chromosome condensing proteins. The chromosomes decondense and nuclear membranes get re-synthesized. Cells then proceed forward into telophase where cytokinesis takes place and the cell cycle completes.
Learn more about
References
- Bartek J, Lukas J. (2001). Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol, 13(6), 738-747. doi:10.1016/S0955-0674(00)00280-5
- Vermeulen K, Van Bockstaele DR, Berneman ZN. (2003). The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif, 36(3), 131-149. doi:10.1046/j.1365-2184.2003.00266.x
- Cross FR, Buchler NE, Skotheim JM. (2011). Evolution of networks and sequences in eukaryotic cell cycle control. Philos Trans R Soc Lond B Biol Sci, 366(1584), 3532-3544. doi:10.1098/rstb.2011.0078
- Morgan DO. (1997). Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol, 13, 261-291. doi:10.1146/annurev.cellbio.13.1.261
- Martín Y, Domínguez-Kelly R, Freire R. (2011). Novel insights into maintaining genomic integrity: Wee1 regulating Mus81/Eme1. Cell Div, 6, 2-5. doi:10.1186/1747-1028-6-21
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. (1993). Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J, 12(1), 53-63. https://pubmed.ncbi.nlm.nih.gov/8428594.
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. (1998). An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell, 93(6), 1067-1076. doi:10.1016/S0092-8674(00)81211-8
Abbreviations
APC/C: Anaphase promoting complex/cyclosome
ATM: Ataxia telangiectasia mutated kinase
ATP: Adenosine triphosphate
ATR: Ataxia telangiectasia related kinase
Cdc20: Cell division cycle protein 20
Cdc25: Cell division cycle protein 25
CDKs: Cyclin-dependent kinases
Chk: Checkpoint kinase
DNA: Deoxyribonucleic acid
Mad2: Mitotic arrest deficient 2
Mdm2: Murine double minute 2
MPF: Maturation-promoting factor
PLK1: Polo-like kinase 1
Rb: Retinoblastoma
SCF: Skp 1-Cul 1-Fbox protein