What is an Amino acid?
Amino acids are chemical molecules that combine to produce proteins; thus, they are known as protein components. Peptides and proteins are both made up of long chains of amino acids.
- These biomolecules participate in a variety of biological and chemical functions in the human body and are required for human growth and development.
- They are required for the synthesis of enzymes, hormones, and neurotransmitters. And are also engaged in a variety of metabolic pathways within cells all over the body.
- They are chemical molecules that contain basic amino groups (-NH2) as well as carboxyl groups. (-COOH).
- There are around 300 amino acids found in nature. There are 20 of them in total that are used in the building of proteins.
Structure of Amino acid
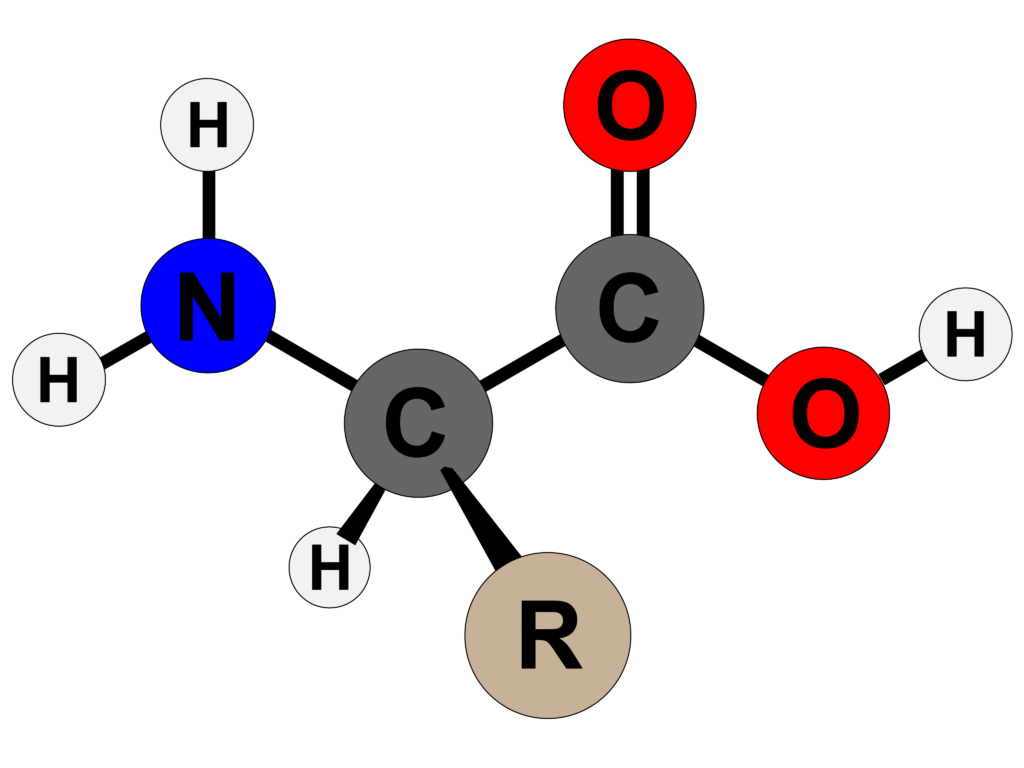
- The general structure of amino acid can be written as H2NCH RCOOH
- There are 20 naturally occurring amino acids, all of which share three structural features: an amino group (-NH3+), a carboxylate (-COO-) group, and a hydrogen atom.
- They differ from one another in their R group side-chain.
- Each amino acid contains 4 distinct groups linked to α – carbon. And these 4 groups are amino group, COOH, hydrogen atom and sidechain(R).
What are the different types of amino acid?
To function properly, our bodies require 20 different types of amino acids. These 20 amino acids combine in various ways to form proteins in our bodies.
- Out of 20 amino acids, our body can easily synthesize a few on its own, which are called non-essential amino acids. These are alanine, asparagine, arginine, aspartic acid, glutamic acid, cysteine, glutamine, proline, glycine, serine, and tyrosine.
- Aside from these, there are nine other amino acids that are essential amino acids since our bodies cannot produce them. Isoleucine, histidine, lysine, leucine, phenylalanine, tryptophan, methionine, threonine, and valine are examples of essential amino acids.
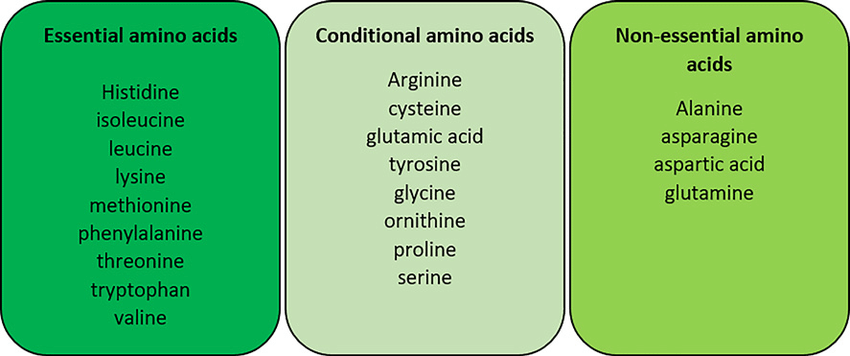
Essential amino acids
our body cannot produce them; supplemented through the food we eat.
Source of Essential amino acid
- Animal proteins, such as meat, eggs, and poultry, are the best source of essential amino acids.
- However, some plant food, such as edamame and tofu made from soy, contain all nine essential amino acids. This means they are “complete” protein sources.
The 9 essential amino acids are:
Histidine
- Histidine was isolated in 1896, and its structure was verified in 1911 through chemical synthesis.
- Histidine is a direct precursor of histamine and an important carbon source in purine synthesis.
- Histidine’s side chain can operate as a proton acceptor and donor when incorporated into proteins, providing essential features when paired with enzymes such as chymotrypsin and those involved in the metabolism of carbohydrates, proteins, and nucleic acids.
- Histidine helps make a brain chemical (neurotransmitter) called histamine. Histamine is essential for our body’s immune system, digestion, sleep, and sexual function.
Isoleucine
- In 1904 isoleucine was isolated from beet sugar molasses.
- The hydrophobic side chain of isoleucine plays an essential role in determining the tertiary structure of proteins that contain it.
- Isoleucine is involved in muscle metabolism and immunological function in our body.
- It also aids in the production of hemoglobin and the regulation of energy levels in the body.
Leucine
- In 1819, leucine was isolated from cheese, and from muscle and wool in its crystalline state in 1820. It was developed in a laboratory in 1891.
- Only the l-stereoisomer is found in mammalian protein and can be degraded by the body’s enzymes into simpler molecules.
- Some DNA binding proteins contain sequences with leucine organized in leucine zipper patterns.
- Leucine helps in the production of protein and growth hormones in the body. It also contributes in the growth and regeneration of muscular tissue, the healing of wounds, and the regulation of blood sugar levels.
Lysine
- Lysine was isolated from the milk protein casein for the first time in 1889, and its structure was discovered in 1902.
- Lysine is essential for the binding of enzymes to coenzymes and for the activity of histones.
- Many cereal crops are extremely low in lysine, resulting in deficiencies in some communities that rely mostly on them for food, as well as in vegetarians and low-fat dieters. Consequently, efforts have been made to develop corn strains rich in lysine.
- Lysine is required for the synthesis of hormones and energy. It is also necessary for calcium and immunological function.
Methionine
- In 1922, methionine was isolated from the milk protein casein, and its structure was identified in 1928 by laboratory synthesis.
- Methionine is a significant sulfur source for a variety of molecules in the body, including cysteine and taurine.
- Methionine, due to its sulfur concentration, serves to prevent fat formation in the liver and detoxify metabolic wastes and toxins.
- Because methionine is the only necessary amino acid that is not present in soybeans, it is synthesized commercially and added to many soy meal products.
- Methionine aids in tissue growth, metabolism, and detoxification in the body. Methionine helps with the absorption of important minerals, including zinc and selenium.
Phenylalanine
- In 1879, phenylalanine was isolated from a natural source (lupine sprouts) and chemically synthesized in 1882.
- The human body can normally convert phenylalanine to tyrosine, but in people with the genetic disorder phenylketonuria (PKU), the enzyme that performs this conversion is inactive. If left untreated, phenylalanine accumulates in the blood, causing mental retardation in children.
- Although one in every 10,000 children is born with the illness, adopting a low-phenylalanine diet early in infancy can help reduce the symptoms.
- Phenylalanine is required for the production of chemical messengers in the brain, such as dopamine, adrenaline, and norepinephrine. It is also required for the synthesis of other amino acids.
Threonine
- In 1935, threonine was isolated from fibrin and synthesized in the same year. Only the l-stereoisomer is found in mammalian proteins and is relatively unreactive.
- Although important in many reactions in bacteria, its metabolic role in higher animals, including humans, remains unclear.
- Threonine is essential for the formation of collagen and elastin. These proteins provide our skin and connective tissue structure.
- They also help in the formation of blood clots, which aid in the prevention of bleeding. Threonine is essential for fat metabolism as well as immunological function.
Tryptophan
- Tryptophan was isolated from casein (milk protein) in 1901 and its structure was identified in 1907, however only the l-stereoisomer is found in mammalian proteins.
- Bacteria in the human gut degrade dietary tryptophan, generating chemicals such as skatole and indole, which give feces their disagreeable odor.
- Tryptophan is converted to vitamin B3 (nicotinic acid or niacin) at a pace that is insufficient to keep us healthy.Consequently, we must also ingest vitamin B3, failure to do so leading to a deficiency called pellagra.
- Tryptophan helps in the maintenance of our body’s correct nitrogen balance. It also contributes to the production of serotonin, a brain chemical (neurotransmitter). Serotonin is a neurotransmitter that regulates our mood, appetite, and sleep.
Valine
- After being isolated from albumin in 1879, the structure of valine was discovered in 1906. In mammalian protein, only the l-stereoisomer is present.
- Valine may be broken into simpler chemicals in the body, however in persons with maple syrup urine illness, a defective enzyme blocks this process, which can be fatal if left untreated.
- Valine is important in muscle growth, tissue regeneration, and energy production.
Non-essential amino acid
- Non-essential acids are created by our bodies naturally and have nothing to do with the foods we eat.
- Non-essential amino acid are alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Source of Non-essential amino acid
- Non-essential amino acids can be taken with food, which is especially helpful for children who are fast growing. They can, however, be synthesized in the human body utilizing the available carbohydrates and amino acids.
- Almost all of these amino acids are synthesized from alpha ketoacids through a chemical process known as transamination.
- Transamination is the process of removing an amino group from a molecule and combining it with a ketoacid to generate a new and distinct amino acid.
The types of non-essential amino acid are given below:
Alanine
It aids in the breakdown and elimination of poisons from the body.
Arginine
Arginine improves blood pressure and blood flow by increasing nitric oxide generation.
Asparagine
It helps for the maintenance of healthy brain cells and the central nervous system.
Aspartic acid
Aspartic acid generates more amino acids and essential enzymes.
Cysteine
Cysteine operates as an antioxidant and provides resistance to our bodies; it is required for the production of collagen. It has an impact on the skin’s texture and elasticity.
Glutamic acid
Glutamic acid acts as a neurotransmitter and is primarily involved in the formation and functioning of the human brain.
Glutamine
Glutamine supports brain health and is required for the production of nucleic acids (DNA and RNA).
Glycine
Glycine helps in the maintenance of healthy cell growth and function, as well as the healing of wounds. It is a neurotransmitter.
Proline
Proline plays a key role in tissue healing through the production of collagen, preventing the thickness and hardening of artery walls (arteriosclerosis), and the regeneration of new skin.
Serine
Serine helps in muscle growth and the production of immune system proteins.
Tyrosine
Tyrosine is required for the formation of thyroid hormones T3 and T4, as well as the synthesis of a class of neurotransmitters and melanin, which are natural colors found in our eyes, hair, and skin.
Difference between
Essential amino acid and Non-essential amino acid
| Parameter | Essential amino acid | Non-essential amino acid |
| Definition | Essential amino acids are those that must be obtained from food since they cannot be produced in the body. | Non-essential amino acids can be produced in the body from other amino acids and carbohydrates. |
| Number | 9 amino acids out of a total of 20 are regarded to be necessary. | 11 of the 20 amino acids are non-essential. |
| Food source | Essential amino acids can be obtained from animal protein sources such as eggs and meat, as well as from vegetable sources such as soy and quinoa. | Nonessential amino acids can be synthesized in the body from other acids and carbohydrates. |
| Function | Essential amino acids help to develop and repair muscles as well as generate neurotransmitters for the nervous system. | Non-essential amino acids play a function in the production of other chemicals as well as a source of energy. |
| Deficiency | A deficiency of essential amino acids can lead to a weakened immune system and a decrease in neurotransmitters in the brain. | A deficiency of nonessential amino acids is relatively rare, but it can occur during starvation or illness; in premature infants, this can lead to an accumulation of ammonia. |
Reference
- https://www.everydayhealth.com/amino-acids/guide/
- https://www.healthline.com/nutrition/essential-amino-acids#what-they-are
- https://byjus.com/biology/amino-acids/
- https://www.britannica.com/science/amino-acid
- https://my.clevelandclinic.org/health/articles/22243-amino-acids
- https://www.technologynetworks.com/applied-sciences/articles/essential-amino-acids-chart-abbreviations-and-structure-324357
- https://www.sciencedirect.com/topics/neuroscience/nonessential-amino-acid
- https://healthyeating.sfgate.com/nonessential-amino-acids-human-nutrition-6079.html
- https://medlineplus.gov/ency/article/002222.htm
- http://www.differencebetween.net/science/health/difference-between-essential-and-nonessential-amino-acid/