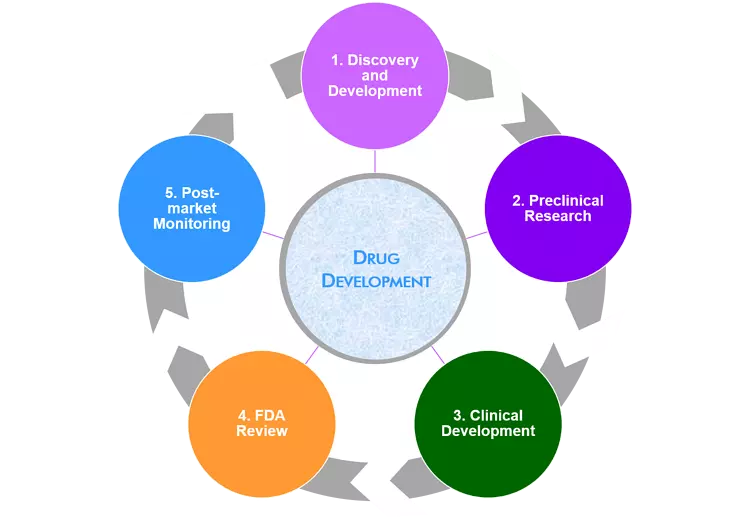
The process of finding novel drugs to treat different illnesses is called drug discovery. Target identification, lead discovery, lead optimization, preclinical testing, clinical trials, and regulatory approval are some of the phases in this lengthy and complex process. We will go over each step of the drug discovery process in the following article and why it is crucial to maintaining human health.
STEPS OF DRUG DISCOVERY AND DEVELOPMENT
Target Identification
- Target identification is the initial phase of drug discovery. A biological target is an element or technique that has an effect in a certain illness.
- Any biological mechanism essential to the development of the disease, whether it be a protein, enzyme, gene, or any other, might be the cause of the disease.
- Potential targets are discovered by scientists using a variety of methods, including genetic and biochemical methodologies, epidemiological research, and clinical observations.
The identification and description of the molecular pathways implicated in a particular disease is done using various genetic and biochemical techniques.
- To do this, researchers may discover the genes that are overexpressed or altered in the disease, as well as the proteins and enzymes that are important in the development of the disease.
- They might also screen huge chemical databases for molecules that interact with the target. In epidemiological investigations, vast datasets are analyzed to find variables, such environmental exposures or genetic risk factors, that are connected to a particular disease.
- Researchers employ cutting-edge methods and instruments to validate targets, including disease association, bioactive compounds, cell-based models, protein interactions, signaling pathways analysis, functional analysis of genes, in vitro genetic manipulation, antibodies, and chemical genomics.
For example, the Sanger Whole Genome CRISPER library and Duolink PLA are excellent sources for drug discovery targets.
- Studying people with a particular illness in order to find possible targets or biomarkers is known as clinical observation.
Lead Discovery
The subsequent stage in drug development is lead discovery, which comes after a potential target has been located. A chemical that interacts with the target and has positive pharmacological action is called a lead compound. Lead discovery can be accomplished using a variety of methods, including as high-throughput screening, Hit to Lead (H2L) method, virtual screening, and natural product screening.
- High-throughput screening includes searching through huge chemical libraries for molecules that interact with the target. Robotic systems that can test tens of thousands of chemicals each day are used for this.
- The Hit to Lead (H2L) method evaluates and optimizes small molecule hits from an HTS in a constrained manner into lead compounds. The method of lead optimization is then applied to these compounds.
- Virtual screening is the process of determining which chemicals are most likely to interact with the target based on its structure and attributes using computer simulations.
- Natural product screening includes looking for molecules that interact with the target in natural goods, such as plant extracts or microbial cultures.
Lead Optimization
Lead optimization is the subsequent phase of drug development after the identification of a lead molecule. To increase the lead compound’s pharmacological activity, selectivity, and safety, the structure must be modified. Lead optimization can be done using a variety of methods, including as medicinal chemistry, structural biology, and pharmacology.
- In medicinal chemistry, novel molecules that have a structural similarity to the lead chemical are synthesized and tested. The lead compound’s chemical structure may need to be slightly altered in order to increase potency and selectivity.
- In structural biology, the three-dimensional structure of the target and how it interacts with the lead molecule are ascertained using X-ray crystallography and other methods. Designing novel substances that modulate the target more successfully can be done using this knowledge.
- Pharmacology involves investigating the lead compound’s pharmacokinetic, pharmacodynamic, and safety characteristics in preclinical models.
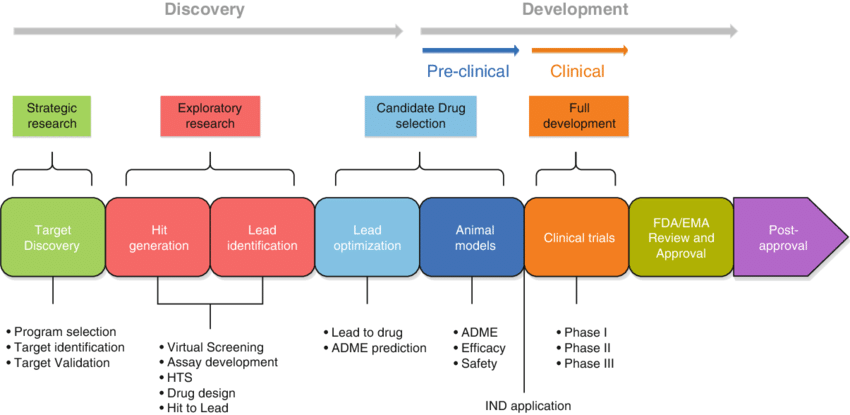
Preclinical Testing
- Preclinical testing is necessary before a lead chemical can be evaluated on humans.
- Preclinical testing entails examining the drug’s pharmacokinetics, effectiveness, and safety in animal models.
- Preclinical testing aims to spot any possible safety issues and establish the best dose and dosing schedule for the compound in question.
These experiments are carried out in vitro and in vivo by scientists using unlimited doses.
Ex vivo, In Vivo, and in vitro tests
These three study kinds include either employing full, live creatures or cells, such as those from animals or people; or using non-living organisms or tissue extract.
- Examples of in vivo preclinical research include the creation of novel medications utilizing animal models such as mice, rats, and dogs. In vitro research is lab-based research.
- Ex vivo employs animal tissues or cells from an inanimate animal. Ex vivo research tests include discovering effective cancer treatments, measuring tissue attributes (physical, thermal, electrical, and optical), and creating accurate models for novel surgical techniques. For tiny explant cultures that offer a dynamic, regulated, and sterile environment, an ex vivo test always starts with a cell.
In-Silico Assays
On a computer or through computer simulation, in silico assays are test systems or biological tests. These are anticipated to grow in popularity as computer power and our behavioral knowledge of molecular dynamics and cell biology continue to advance.
Clinical Trials in Drug Development
- Clinical trials are a crucial part of the process of developing novel drugs.
- They are made to examine a prospective new medication’s pharmacokinetics, effectiveness, and safety in human test participants.
- Phases of clinical trials are carried out, with each phase intended to address particular questions regarding the safety and effectiveness of the medicine.
The many stages of clinical trials and their significance in drug development will be described below.
Phase I Clinical Trials – Healthy Volunteer Study
- Phase I clinical trials are the initial step of a possible new drug’s clinical testing.
- These tests are often carried out on healthy volunteers to assess the drug’s pharmacokinetics, safety, and tolerability.
- Phase I clinical trials’ main goals are to ascertain the drug’s maximum tolerable dose and to pinpoint any negative effects or side effects.
- Small volunteer groups, usually between 20 and 80 people, are used for phase I clinical studies.
- Depending on the pharmacokinetics of the medicine and the number of doses being evaluated, these studies may continue for a number of weeks or months.
- Participants in phase I clinical trials are constantly watched for side effects and regularly tested for blood and urine to assess the pharmacokinetics of the medicine.
Phase II Clinical Trials- Studies In Patient Population
- Phase II clinical trials are the second stage of a possible new drug’s clinical testing.
- Usually, just a few people with the target disease or condition are used in these trials.
- Phase II clinical trials’ main goal is to assess the drug’s effectiveness and safety in human subjects.
- Phase II clinical studies might involve several hundred individuals and are often larger than phase I trials.
- Depending on the effectiveness of the treatment and the number of dosages being evaluated, these studies may run several months to a few years.
- Phase II clinical trial participants are constantly watched for side effects and regularly subjected to imaging and blood testing to gauge the drug’s effectiveness.
Phase III Clinical Trials
- Phase III clinical trials are the last step of a possible new drug’s clinical testing.
- The target ailment or condition is often tested in these studies on a sizable number of people.
- Phase III clinical trials’ main goals are to determine any unusual or long-term side effects and to establish the medication’s effectiveness and safety in a broader patient group.
- Phase III clinical studies can involve thousands of people and are often substantially bigger than phase II trials.
- To guarantee a broad patient group, these studies frequently extend many years and take place at numerous different locations.
- Participants in phase III clinical trials are rigorously watched for side effects and regularly subjected to imaging exams, blood tests, and other evaluations to determine the effectiveness and safety of the medication.
Regulatory Approval in Drug discovery
- The data is sent to regulatory organizations, such as the European Medicines Agency (EMA)or the U.S. Food and medicine Administration (FDA), after a medicine has successfully completed phase III clinical trials.
- These organizations examine the information to decide if the medicine is effective and safe, and if the advantages exceed the drawbacks.
If the medication is authorized, it may be promoted and sold to customers.
Post-Marketing Surveillance
- Continuous monitoring is necessary to guarantee a drug’s efficacy and safety even after it has been authorized and placed on the market.
- Post-marketing surveillance or pharmacovigilance is the term used for this. Monitoring the drug’s safety and effectiveness in a wider patient population during post-marketing surveillance entails looking for any unusual or persistent side effects that were undetected during clinical studies.
Conclusion
Drug research and discovery depend heavily on clinical trials. They are intended to assess a possible new drug’s pharmacokinetics, effectiveness, and safety in human subjects. Each step of clinical trials is intended to address a particular query concerning the safety and effectiveness of the medicine. The results of clinical studies are used to assess a drug’s efficacy and safety, as well as its suitability for patient marketing. Even once a medicine is licensed, ongoing monitoring is necessary to guarantee its efficacy and safety.