One of the main causes of mortality in the world is cancer. Several traditional cytotoxic methods for treating neoplastic illnesses have been developed throughout the years. Yet, due to their limited efficacy in light of the variety of cancer cells, there is a continuous quest for treatment strategies that provide better results, such as immunotherapy, which makes use of and boosts the immune system’s normal functioning.
Surgery, chemotherapy, and radiation therapy have been the cornerstones of cancer treatment for many years. They remain essential therapy principles, although new categories of treatment have lately contributed to a change in the therapeutic landscape for cancer patients.
Immune checkpoint inhibitors, for instance, are already widely used to treat a variety of cancers, including lymphoma, melanoma, lung, kidney, and bladder cancer.
The Food and Drug Administration has authorized six CAR T-cell treatments since 2017. (FDA). All of them have been given the go-ahead to treat blood diseases such lymphomas, certain varieties of leukemia, and most recently multiple myeloma.
Learn more about Cancer and its causes
What is CAR T-cell therapy?
- Chimeric antigen receptor (CAR) T-cell therapy is the process of finding, attacking, and destroying cancer cells effectively utilizing specially modified T cells. It is the immunotherapeutic process.
- This technique uses the body’s own immune system.
- Blood is drawn to get a sample of the patient’s T cells, which are subsequently altered to develop unique features on their surface known as chimeric antigen receptors (CARs).
- These CAR T cells may now attach to a particular antigen on the patient’s cancer cells and destroy them when reinfused into the patient.
What are T cells?
White blood cell subtype. T cells are produced by stem cells in the bone marrow and are a vital part of the immune system. They may assist in the fight against cancer and protect the body from infection. Also known as a thymocyte and a T lymphocyte.
CAR T Therapy Process
- Collection of T cells from patient
T cells are obtained by the process of apheresis, which comprises drawing blood from the body and removing one or more blood components (such as plasma, platelets, or white blood cells). Remaining blood is administered back to the patient’s body.
2. Genetically modifying T cells in laboratory
The T cells are sent to a lab or a pharmaceutical manufacturing facility, where they are genetically modified by inserting DNA to create chimeric antigen receptors (CARs) on their surface. This reengineered T cells are now called CAR T cells, Proteins called CARs enable T lymphocytes to detect an antigen on selected tumor cells. CAR T cells are then grown and expanded in the laboratory.
This T-cell genetic alteration may take place either using nonviral gene transfer techniques such DNA-based transposons, CRISPR/Cas9 technologies, or direct electroporation of in vitro produced mRNA.
3. Infusion of CAR T cells in the patient’s body
Before the infusion of CAR T cells, chemotherapy is given to the patient to lower the immune system. It increases the likelihood that CAR T cells will get activated and begin the cancer-fighting process. The CAR T cells begin to multiply as soon as they begin binding with cancer cells, and they have the potential to help in the destruction of even more cancer cells.

What types of cancer are treated with CAR T-cell therapy?
Many CAR T-cell therapies have been licensed by the U.S. Food and Drug Administration (FDA) for patients with certain blood malignancies that don’t respond to chemotherapy and other treatments. The treatment of blood cancer in patients who have had prior effective therapies is also done with this medication.
The malignancies that are currently being treated using CAR T-cell therapy are listed below:
- B-cell acute lymphoblastic leukemia (ALL): This malignancy affects developing white blood cells called B lymphocytes while they are still in the bone marrow.
- B-cell non-Hodgkin lymphoma, including: Diffuse large B-cell lymphoma (DLBCL), Follicular lymphoma with DLBCL, High-grade B-cell lymphoma.
- Primary mediastinal large B-cell lymphoma.
- Mantle cell lymphoma
- Multiple myeloma: This is a cancer of plasma cells, the antibody-producing cells.
- Relapsed or refractory follicular lymphoma
- Relapsed or refractory multiple myeloma
- Relapsed or refractory acute lymphoblastic leukemia
Approved CAR T-cell therapies
The US Food and Drug Administration (FDA) has given the green light for CAR T-cell treatments to treat certain leukemias, lymphomas, and multiple myeloma cases. Once alternative forms of treatment have been exhausted, CAR T-cell therapy is frequently employed.
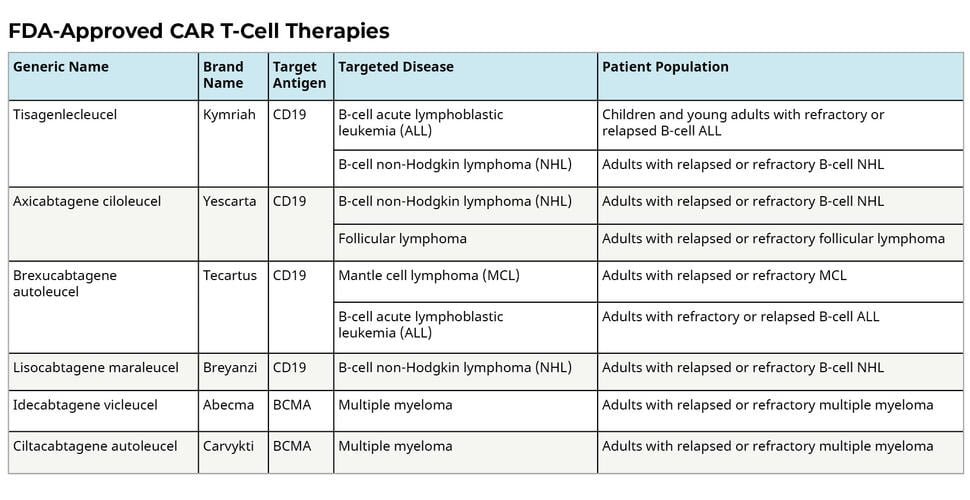
Source: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
Side effects of CAR T – cell therapy
When used to treat some types of cancers that are difficult to cure, CAR T-cell therapy can be highly successful. Nevertheless, it can occasionally have terrible, even fatal, adverse effects.
- Cytokine release syndrome (CRS)
CAR T cells have the ability to start a large-scale release of cytokines, which results in the inflammatory state known as cytokine-release syndrome (CRS). When CAR T cells proliferate in the body and destroy cancer cells, cytokines (chemical messengers that assist T cells in performing their duties) are created.
Flu-like symptoms might include a high temperature, chills, low blood pressure, breathing difficulties, or disorientation. These signs might be minor or major.
2. Nervous system problems
Neurotoxicity, a condition that CAR T-cells can occasionally induce, is a concern for the brain. Confusion, difficulties with language and speech, and stupor are among potential symptoms that patients may encounter.
3. Increased risk of infection
CAR T-cells are created to recognize the protein CD 19 and are used to treat certain leukemias and lymphomas. Most B lymphocytes have CD 19 on their surface. White blood cells called B cells are crucial in the fight against infection, just like T cells.
The CD 19 protein-targeting CAR T-cell treatment also kills B cells. Both healthy and malignant B cells are killed by it. This either decreases the amount of B cells or completely eradicates them. Your ability to fight infections is hampered as a result.
This adverse effect may require medical attention. Immunoglobulin therapy is the name of this procedure. It has antibodies that might assist in battling illness.
Limitations and the future of CAR T cell therapy
The clinical trials have generated impressive results with the patients of blood cancers. Research is underway in using CAR-T for the treatment of solid tumors.
References
- https://www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/faq-about-car-t-cell-therapy/
- https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- https://pubmed.ncbi.nlm.nih.gov/29667553/
- https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/CAR-T-cell-therapy
- https://my.clevelandclinic.org/health/treatments/17726-car-t-cell-therapy
- https://www.pennmedicine.org/cancer/navigating-cancer-care/treatment-types/immunotherapy/what-is-car-t-therapy
- https://www.lls.org/treatment/types-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/t-cell