What is Flow cytometry?
Flow cytometry is the measuring (metry) of cells (cyto) as they flow (cells in motion) past a detecting device. The technique of flow cytometry is used to evaluate cells for a number of functions, such as cell counting, phenotyping, cell cycle analysis, and viability.
A flow cytometer uses lasers to create light, which is subsequently dispersed by the sample’s cells, recorded by detectors, and converted into signals for analysis and measurement.
It enables the multiparametric simultaneous investigation of the physical and chemical properties of up to a million particles per second. As a result, it can analyze and purify cells in suspension quickly and quantitatively.
Principle of Flow cytometry
The main principle of flow cytometry is the single-file passage of cells in front of a laser for detection, counting, and sorting. Fluorescently labeled cell components are activated by the laser to produce light at various wavelengths.
It utilizes the principle of Laminar flow sheath. This happens when a stream of fluid that is flowing more quickly is injected slowly under pressure with a monodispersed suspension of particles (cells). This serves as the foundation for the fluidic system employing hydrodynamic focusing, creating a “sheath” that surrounds and aligns the cells. The cells flow through an aperture along a path that is nearly similar to one another, passing past a laser’s intense focus point where optical and/or electrical signals reflect certain cell biologic qualities (size, volume, internal characteristics).
The quantity and type of cells in a sample may then be determined by measuring the fluorescence.
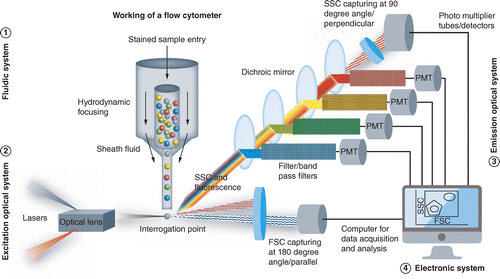
Three systems of Flow cytometry
The main three systems of flow cytometry are: Fluidics, Optics and Electronics
1. Fluidics:
Particles are sent in a stream by the fluidics system to the laser beam for analysis.
- Sheath Fluid: Sheath fluid, which is typically a buffered saline solution, passes through a flow cytometer in laminar flow.
- Laminar flow: A flow in which there is minimal to no mixing between the liquid layers as they pass one another.
2. Optics:
Lasers are used in the optics system to illuminate the sample stream’s particles, and optical filters are used to guide the generated light signals to the proper detectors.
As the cells pass the laser one at a time, their cell structure causes some of the beam to be refracted. While other detectors are positioned at a 90-degree angle to measure the side-refracted light, some of the light refracts beyond the cell to a detector behind it.
Forward Scatter (FSC) —
- FSC is useful in determining size of the cell. The relative cell size is commonly determined by light scattering past the cell.
- The diameter and measured intensity on the light scatter detector are correlated.
- One situation where forward scatter is useful is in the differentiation of size of white blood cells.
Average Diameter
- Lymphocyte: 8.5 micrometers.
- Neutrophils: 8.85 micrometers
- Monocytes: 15 micrometers
- Eosinophil: 14.5 micrometers
- Basophils: 15 micrometers
Knowing the size using FSC won’t reveal the type of blood cell. Hence, it only does partial differentiation of cell. Results from FSC and SSC must be combined in order to completely identify.
Side Scatter (SSC):
- Light that is scattered perpendicularly (at an angle of 90 degrees) is filtered by a number of lenses and mirrors before passing through photomultipliers (PMTs), where the data points are gathered.
- SSC serves to offer information on the complexity of the cell, whereas FSC measures the diameter and volume of the cells. The nucleus structure and granularity of the cell may be detected by SSC.
- The ability to differentiate between cells can be greatly increased by combining these two detection techniques.
3. Electronics:
The electronics system transforms the observed light signals into computer-processable electrical impulses. The electronics system can also start making sorting judgments to charge and deflect particles for some instruments having a sorting capability (FACS).

Flow cytometry staining protocol
- After cell harvesting, aliquot up to 1 x 106 cells/100 L into FACS tubes. Fc-block cells were incubated for 15 minutes at room temperature with blocking IgG
- Since low temperature and the presence of sodium azide prevent the modification and internalization of surface antigens, which might result in a reduction of fluorescence intensity, staining with ice cold reagents/solutions and at 4°C is advised. Restoring cell function, such as if cells are to be collected for functional experiments, adding sodium azide to buffers must be avoided. It prevents the metabolic process.
- Add a previously titrated quantity of conjugated primary antibody (5–10 L/106 cells), then vortex. Cells should be let to sit at room temperature in the dark for 30 minutes.
- Wash the cells in 2 mL of Flow Cytometry Staining Buffer to remove any unattached antibodies. Centrifuge the suspended cells for 5 minutes at 350-300 x g while decanting the buffer. By adding 2 mL of Flow Cytometry Staining Buffer, the cells are once again suspended. Two times through this washing process.
- To resuspend cells for use in the final flow cytometric analysis, use 200–400 L of flow cytometry staining buffer.
Applications of Flow cytometry
There are several methods and applications for flow cytometry that are appropriate for use in a variety of academic disciplines. In research, flow cytometry is specifically utilized for a variety of reasons, such as:
- Cell Counting and Characterization: Using surface markers, size, and shape, flow cytometry may be utilized to count and categorize various cell kinds and populations.
- Analysis of cell surface markers: To investigate the behavior and interactions of cells, flow cytometry may be used to locate and measure cell surface markers, such as antigens and receptors.
- Cell sorting: Using flow cytometry, certain cell populations may be separated and purified depending on their surface markers or other factors, enabling additional research or testing.
- Multiparameter analysis:It may assess numerous parameters concurrently on a single cell, giving complicated biological material a thorough examination.
- Detection of aberrant cells: It can be used to detect and quantify the aberrant or abnormal cells.
- Investigation of immune cell function: Using flow cytometry, researchers may examine how immune cells produce cytokines, engage in phagocytosis, and move across the body.
- Cell signaling analysis: It may be used to examine how particular signaling pathways are activated in response to different stimuli, offering crucial insights into cellular processes.
- Hematological illness diagnosis and monitoring: It is frequently used in hematology to diagnose and keep track of a variety of blood disorders, such as anemia, leukemias, and lymphomas.
- Cell biology research: Cell activity is studied using flow cytometry, which offers crucial insights into biological processes such cell proliferation, differentiation, and apoptosis.
Differences between Flow Cytometry and FACS
- FACS is a process by which a sample mixture of cells is sorted according to their light scattering and fluorescence characteristics into two or more containers.
- Flow cytometry is a methodology which is utilized during analysis of a heterogeneous population of cells according to different cell surface molecules, size and volume which allows the investigation of individual cells.
Limitations of Flow & FACS
- Highly specific, highly multiplexed measurements are required, however, can’t fully eliminate antibody cross-talk and fluorescence bleed-through
- good flow-validated or FACS-validated antibody against the target of interest is hard to be found
- Assay difficult to work with intracellular protein
- Large number of cells are required in the sample.
- https://nanocellect.com/blog/flow-cytometry-vs-facs/
- https://www.bio-techne.com/resources/instrument-applications/flow-cytometry-and-facs-cell-sorting-milo
- https://www.rndsystems.com/resources/protocols/flow-cytometry-protocol-staining-membrane-associated-proteins-suspended-cells
- https://medicine.yale.edu/immuno/flowcore/protocols/analysis/
- https://nanocellect.com/blog/breaking-down-the-principles-of-flow-cytometry/
https://nanocellect.com/blog/flow-cytometry-vs-facs/