What is Transfection?
Transfection is a technique for introducing foreign genetic material (nucleic acid) into eukaryotic cells, which has the potential to change the genetic make-up of host cell.
Deoxyribonucleic acids (DNAs), ribonucleic acids (RNAs), short, non-coding RNAs (siRNA, shRNA, and miRNA), and other forms of genetic material can all be transfected into mammalian cells.
Transfection can be carried out via physical, chemical, or biological means, which are discussed further in the article. It enables us to study how genes, their products, and cell’s function.
TYPE OF TRANSFECTION
Depending on the period of the transgene’s expression in the host cell, transfection can be divided into two categories: Transient and Stable.
TRANSIENT TRANSFECTION:
- Transient transfection refers to maintaining short-term expression of a transgene.
- Nucleic acids do not need to be integrated into the host cell’s genome for transient transfection. A plasmid or oligonucleotides can be used to transfect nucleic acids.
- As host cells divide, transgenic expression will consequently finally disappear. Cells are harvested within 24-72 hrs. for the study.
- The use of transient transfection is frequently used in short-term research to examine the outcomes of a certain gene’s knock-in or knock-down. Isolating RNA or protein for enzymatic activity tests or immunoassays may be necessary for the analysis of gene products.
STABLE TRANSFECTION:
- Stable transfection refers to maintaining long-term expression of a transgene.
- It requires incorporation of foreign DNA into the host nuclear genome or keeping an episomal vector in the host nucleus as an extra-chromosomal element.
- Selective screening is required to distinguish between cells that have not been transfected and those that have acquired foreign DNA. When the transfected DNA has an appropriate drug-resistance marker, this screening can be carried out via drug selection.
- Using such a vector that has a critical gene that is disrupted in a specific cell line is an alternate approach.
- As cells replicate, the transgene may still be produced constitutively.
- Long-term genetic and pharmacological investigations requiring large-scale protein synthesis, studies into long-term gene regulation, the creation of stable cell lines, and gene therapy all benefit from stable transfection.
- Plasmids can randomly integrate, transposases or viruses can actively integrate at random locations, or genome editing technologies like CRISPR can precisely integrate at a certain spot.
METHODS OF TRANSFECTION
Viral delivery, lipofection, electroporation, and calcium phosphate precipitation are a few of the frequently utilized transfection methods. These techniques can also be applied in co-transfections. These methods, which are frequently employed to create stable transfections, entail the simultaneous delivery of two different nucleic acids into the same cell. The transfer of nucleic acids into cells via biolistic delivery systems and in vivo transfection procedures are two examples of how transfection techniques have developed. In vivo transfection protocols enable the systemic administration of siRNA molecules.
Not all of these techniques can be used for all cell types and applications. Each method has benefits and drawbacks; thus, the best approach depends on the goal and design of the experiment. The effectiveness of transfection, cell toxicity, impact on normal physiology, degree of gene expression, etc. are all seen to vary greatly as a result. The best method should be chosen based on the kind of cell and the demands of the experiment, and it should have a high transfection efficiency, low cell toxicity, little impact on normal physiology, be simple to use, and be reproducible.
ELECTROPORATION
- High-voltage electric shock can be used to introduce additional molecules into cells. The process is referred to as electroporation.
- A transient but reversible rupture in the permeability of cells may be caused by an external electric field that is stronger than the capacitance of the cell membrane. Different chemicals can go across the membrane when it is in this brief condition and enter the cell. Either simple diffusion or electrophoretic transit through the cell membrane constitutes the translocation.
- Conventional electroporation may be altered to solely influence the organelle membranes and leave the cell membrane alone.
- Very quick, very high-voltage electric impulses can be used to accomplish this. Different pulse amplitudes can be used in electroporation to alter the area of the cell membrane’s permeabilization (the higher the amplitude, the more area permeabilization issues there are), whereas pulse length or the number of pulses affect the degree of permeabilization. Smaller molecules are absorbed without regard to charge because they are diffused within the cell.
- This technique is frequently used to transfect cells that are challenging to transfect, such as primary cells, stem cells, and B cell lines.
- High voltage usage, however, may result in cell necrosis, apoptosis, and irreversible cell damage.
CALCIUM PHOSPHATE TRANSFECTION
- The transport of material across the negatively charged cell membrane is necessary for transfection. As nucleic acids like DNA and RNA are negatively charged as well, they reject one another and prevent the cell from absorbing them. Using positively charged carrier molecules to transport negatively charged substrates in close proximity to the cell membrane, where they may be absorbed by endocytosis, is one technique to get around this problem. One of the chemical transfection techniques utilizing this idea is calcium phosphate transfection.
- According to the technique, calcium chloride and DNA must be combined before being carefully added to a buffered saline/phosphate solution and allowed to sit at room temperature. A precipitate is produced by the regulated addition and is applied to the grown cells. Endocytosis or phagocytosis are used by cells to take up the precipitate. For both temporary and stable transfection of a range of cell types, calcium phosphate transfection is often utilized.
- Yet, because of its unpredictability, calcium phosphate co-precipitation is unsuitable for in vivo gene transfer into whole animals. Furthermore, buffer-salt concentration, pH, temperature, and tiny pH variations (0.1) might reduce the effectiveness of transfection.
- Chemical reagents have been mainly replaced by synthetic lipid-based reagents because of their disadvantages.
LIPOSOME-MEDIATED TRANSFECTION
- Lipid bilayers that form colloidal particles in an aqueous media are referred to as “liposomes.”
- The negatively charged phosphates on the nucleic acid form an association with the cationic head group of the lipid molecule.
- In vitro and in vivo applications, successful delivery of DNA of all sizes, from oligonucleotides to yeast artificial chromosomes, delivery of RNA, and delivery of protein are just a few of the benefits of liposome-mediated delivery.
- Other benefits include relatively high gene transfer efficiency, the ability to transfect some cell types that are resistant to calcium phosphate or DEAE-dextran, and in vitro and in vivo applications.
- Liposome-based methods of cell transfection can be utilized for both short-term investigations of transient expression and long-term research that rely on DNA chromosomal integration or episomal maintenance.
Non-liposomal Reagents
- Although liposomal transfection reagents have many uses, not all cell types may respond well to them.
- Liposome mediated transfection techniques can be substituted with non-liposomal transfection reagents.
- Several kinds of lipids that may form micelles in aqueous solutions and polymers that can form complexes with DNA or RNA are examples of such reagents.
- They offer minimal toxicity, repeatable results, excellent effectiveness, and suitability for a range of cell types, just as liposomal transfection reagents.
- A development of this transfection technique that is particularly well suited for the administration of small-molecule medications in clinical research and therapeutic applications is the use of lipid nanoparticles.
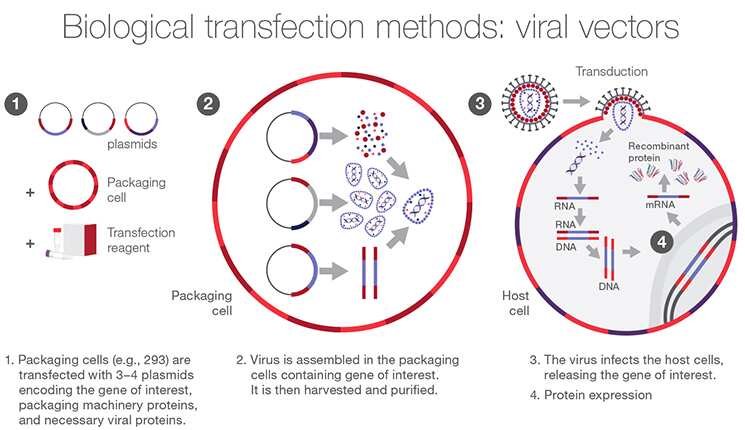
VIRUS MEDIATED TRANSFECTION (Viral Transfection)
Nucleic acids are delivered into cells via viral vectors in this procedure. Even in difficult-to-transfect cells, viral delivery methods including lentiviral, adenoviral, and oncoretroviral vectors can be employed to transfer nucleic acids.
Although viral delivery techniques are incredibly effective, they can be exceedingly time-consuming. Moreover, containment and strict biosafety level monitoring are necessary for the majority of viruses. It is crucial to take into account a number of limiting criteria before carrying out viral transfections, including the lytic nature of viral vectors, cell line packaging, and host-cell specificity.
Adenoviruses
- Adenoviruses, which feature double-stranded DNA, have been employed in gene delivery. Both dividing and non-dividing cells are capable of being infected by this category of viruses.
- Direct contact with v3 and v5 integrins and attachment to cell surface receptors are the first steps in the initiation of an adenoviral infection. Adenovirus then exits the endosome via receptor-mediated endocytosis and travels to the nucleus, where viral transcription and replication take place. Cell death and the release of viral offspring are caused when the infection cycle is complete.
- Adenoviral DNA does not integrate into the chromosomes of the host cell after infection. In consideration of this, this approach is secure, but persistent protein expression cannot be induced. The introduced genes’ initial expression levels are relatively high, but they rapidly decline within a few weeks.
- Recombinant adenovirus creation and generation should be carried out in a Biosafety Level 2 facility.
Adenoviruses-Related Virus
- The Parvoviridae family of viruses includes the tiny, single-stranded DNA, non-enveloped, replication-defective Adenoviruses-Related or Adeno-Associated Virus (AAV).
- Adeno-associated virus (AAV) reproduction depends on co-infections with other viruses, mostly adenoviruses, as a result of its feature. AAV has been demonstrated to exhibit minimal to no activity loss throughout a broad range of temperatures and pH.
- Recombinant episomal DNA is eventually eliminated when the cell multiplies since it does not integrate into the host genomes.
- As a result, the transgene expression declines, with the rate of decline depending on the cell turnover rate. Recombinant adeno-associated virus may be the best choice for several gene therapy applications, according to this information.
Retroviruses
- RNA viruses belonging to the Retroviridae family retrotranscribe their genomes into DNAs using the enzyme known as reverse transcriptase.
- With the help of existing enhancers and other regulatory components that control the expression of viral genes, retro-transcribed DNA can be incorporated into the genome of the host cell.
- A persistent genetic change of the host cell always results from a successful infection or, in the case of vectors, transduction.
- The integration of the transduced genome can result in the deregulation of proto-oncogene expression, which has long been recognized as a driver for retroviral genotoxicity. This poses a significant issue that has emerged as a priority in the development of retrovirals.
- Nevertheless, researchers primarily focus on four families of vectors: lentiviruses (Human Immunodeficiency Virus, HIV), gammaretroviruses (Murine Leukemia Virus, MLV), spumaviruses (Human Foamy Virus, HFV), and alpharetroviruses.
– Lentiviruses (HIV)
are widely used due to their ability to infect both dividing and non-dividing cells.
Learn more on Lentivirus Production : https://thesciencenotes.com/lentivirus-production-and-concentration/
– Gammaretroviruses (MLV)
can be employed to infect cells considered as resistant to non-lentiviral vectors. They can deliver genes to non-dividing cells.
– Spumaviruses (HFV)
can package large transgene cassettes and have desirable safety profile.
– Alphateroviruses
such as Avian Sarcoma Leukosis Virus may be modified to produce self-inactivating vectors (SIN). They are characterized by relatively neutral integration pattern resulting in low genotoxicity.
FACTORS AFFECTING TRANSFECTION EFFICIENCY
Transfection efficiency, for example, varies greatly with the cell type and its physiological condition prior to transfection. Ideally, the cells should be actively growing, healthy, and free of contamination.
Cell Health
- Cells should be grown in medium appropriate for the cell line and supplemented with serum or growth factors as needed for viability.
- Never use infected cells or medium for transfection, such as those that have been exposed to yeast or Mycoplasma.
- If any of the ingredients, such as thiamine, are chemically unstable, make sure the medium is fresh.
- Media deficient in essential components, such as serum, might impair cell development.
- Incubate cells at 37°C with the proper amount of CO2 (5–10%) and humidity maintained at 100%.
Confluency
- Transfect cells when they are 40–80% confluent.
- Without cell-to-cell contact, a culture with too few cells would develop poorly. When there are too many cells, contact inhibition occurs and makes cells resistant to ingesting foreign DNA.
- Quiescent cells are less effective at absorbing new DNA than actively dividing cells.
Number of Passages
- 50 or less passages should be used.
- In immortalized cell lines, cell properties can alter over time, and cells may react differently to the same transfection circumstances over several passages, leading to subpar expression of the transfected gene.
DNA Quality and Quantity
- Protein, RNA, chemical, and microbial contamination should not be present in the plasmid DNA used for transfections.
- TE buffer or sterile water should be used to dissolve ethanol-precipitated DNA to a final concentration of 0.2-1 mg/ml.
- Depending on the kind of DNA, transfection reagent, target cell line, and number of cells, the ideal amount of DNA to employ in the transfection will change significantly.
References
- Addgene. Transfection Protocols.
- Shao, C., Liu, Y., Ruan, M., Ding, X., Li, Z., Wang, J., … & Zhang, W. (2021). An Overview of Electroporation Applications in Biotechnology. Frontiers in Bioengineering and Biotechnology, 9, 701031. DOI: 10.3389/fbioe.2021.701031
- Thermofisher. Introduction to Transfection.
- Promega. Transfection Basics.
- Sigma-Aldrich. Transfection Reagents.
- Lonza Bioscience. Transfection.
- Bio-Rad Laboratories. Introduction to Transfection.