Lentivirus production is a widely used technique in genetic research and gene therapy. Lentiviruses are a type of retrovirus that can efficiently integrate into the host genome, making them attractive tools for gene delivery. Lentiviral vectors are engineered to carry and express therapeutic genes in target cells, which can be used to treat genetic diseases, cancer, and viral infections. Lentivirus production involves several steps, including the preparation of the viral vector, transfection of packaging cells, and purification of the viral particles. The production process requires careful optimization and validation to ensure high-quality viral vectors with minimal toxicity and off-target effects. Lentivirus production has revolutionized the field of gene therapy and holds great promise for the development of new treatments for a wide range of diseases.
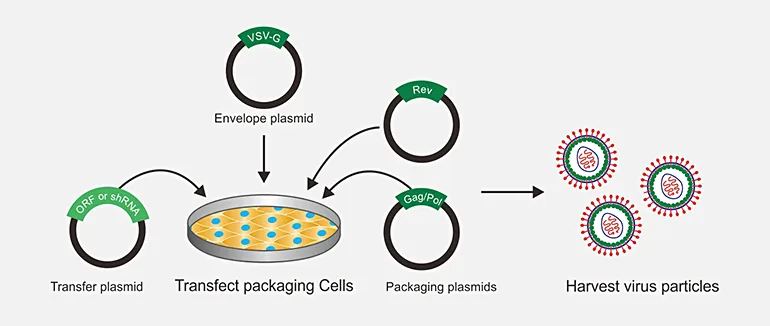
The production and use of lentivirus should be done in appropriate biological safety cabinet. And lentivirus should be disposed after treatment in 10% bleach.
Day 1: Early afternoon (11-12AM)
- Use 293T cells of around 70-80% confluency grown in DMEM high glucose + 10% FBS, 1% Glutamax and 1% penicillin–streptomycin to plate the cells for the transfection.
Cells to be used should be healthy and of low passage number. Cells should reach 90-95% confluency after 24 hours for transfection. (Growth medium without antibiotics can be used for the plating, however, it may or may not affect the efficiency of transfection.) 1 million 293T cells can be plated with 2 ml medium in 6 well plate to obtain 95% confluency the next day. The number of cells to be used should be optimized.
Day 2: 11 AM
- In one tube (A);
250ul opti-mem medium + 7ul Lipofectamine 3000 (Mix well by quick vortexing)
In next tube (B);
250ul opti-mem medium + 6 ul Lipofectamine enhancer + 1.5ug plasmid + 0.3ug PMD2G + 1.2ug PSPAX2
- (Order of addition can be started from smaller volume to larger volume for proper mixing) and mix well by quick vortexing.
- Transfer the mixture in tube A to tube B, QUICK VORTEX, and SHORT SPIN and incubate at room temperature for 15 mins.
- Remove 1ml growth medium (half medium) from the plate.
- Gently transfer the mixture in tube B to the cells from the side of the plate dropwise, gently rocking the plate simultaneously.
- Incubate the plate in 37 degree Celsius incubator with 5% CO2 incubator.
After 5-6 hrs. ,
Change the medium by removing old medium and replace with fresh warm growth medium. Incubate overnight.
Day 3: 24 hrs. post transfection
- Check GFP to calculate the transfection efficiency.
- Harvest the virus. Collect the medium in a sterile tube and store it at 4 degree Celsius and replace with new fresh, warm growth medium.
Day 4: 52 hrs. Post transfection
Harvest the second round of virus. Collect the medium and mix it with the harvest from 24 hrs.
Virus concentration: Viral concentration can be done to get high titer virus using ultracentrifugation.
- Filter the collected virus containing medium through 0.45um filter.
- Centrifuge using a SW-28 rotor @ 25,000 rpm, 90 min, and 4C.
- Discard the supernatant and dissolve the viral pellet with PBS or serum free medium and store at -80 degree Celsius. Avoid multiple freeze thaw by storing in smaller aliquots.
Learn more: