Definition of denaturation of protein
Denaturation is the process of breaking many of the weak bonds, such as hydrogen bonds, that give proteins their highly ordered structure when they are in their native, natural state. Weak and irregularly arranged, denatured proteins are mostly insoluble. Long chains of amino acids, which fold into different three-dimensional structures, make up proteins and are essential to their function.
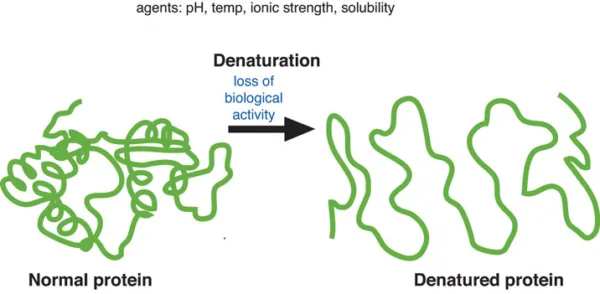
One phenomenon that causes disruptions to the stability and structure of proteins is denaturation. Since proteins are among the most abundant biomolecules in living systems, their chemistry has always been significant. Protein is essential to the basic building blocks of our bodies and their operation. We get this protein from food products like cheese, milk, meat, nuts, pulses, etc.
There are several methods to achieve denaturation, including heating, treating with alkali, acid, urea, or detergents, and vigorous shaking.
Classification of denaturation of protein
The denaturation of proteins can be classified in various ways based on the factors causing the denaturation or the resulting changes. Here are some classifications:
Based on causative factors
- Heat-Induced Denaturation: Denaturation caused by high temperatures that breaks weak linkages that keep proteins structurally intact.
- pH Induced Denaturation: pH-induced denaturation of proteins occurs when the surrounding pH disrupts the protein’s native structure, affecting charged residues.
- Chemical denaturation: It is caused by exposure to specific substances that alter the structure of proteins, such as strong acids, bases, detergents, or organic solvents.
- Mechanical denaturation is the result of physical stress or agitation (such as vigorous shaking or stirring) that alters the structure of proteins.
On the basis of Reversibility
- Reversible Denaturation: When the denaturing conditions are removed, some denaturation processes are reversible, allowing the protein to refold and regain its functional structure.
- Irreversible Denaturation: In certain cases, the denaturation causes structural changes that cannot be reversed, leading to a permanent loss of the protein’s function.
Based on structural changes
- Partial Denaturation: Sometimes a protein will only unfold or change structurally in certain areas.
- Complete protein denaturation: This occurs when the protein completely loses its original structure.
Based on biological impacts
- Functional denaturation is the loss of function caused by structural alterations in a protein.
- Aggregate Formation: Denatured proteins may clump or form aggregates, which will cause them to lose their biological activity and solubility.
Causes of denaturation of protein
The stability of protein and its structure depends on physical and chemical conditions.
- Heat: The weak chemical bonds (van der Waals forces, hydrogen bonds) that keep proteins structurally stable can be broken by high temperatures.
- pH changes: Severe variations in the acidity or alkalinity of the pH can cause denaturation by disturbing the ionic interactions inside the protein.
- Chemicals: Certain chemicals, such as strong acids, bases, detergents, or organic solvents, can disrupt the protein’s structure.
- Mechanical Agitation: By changing the structure of the protein, vigorous stirring or shaking can also result in denaturation.
A protein loses its natural shape and function when it denatures. The amount and type of denaturation determine whether this is irreversible or reversible. When the denaturing conditions are removed, some proteins can refold into their functional form. Nevertheless, in certain instances, denaturation may be irreversible, resulting in the protein’s biological activity being lost. Denatured proteins lose their solubility and functionality and frequently clump and aggregate.
Process of denaturation of protein
Proteins’ secondary, tertiary, and quaternary structures can be readily changed through a process known as denaturation. These modifications may be highly harmful.
- Denaturation can be brought on by heat, exposure to bases or acids, or even by forceful physical contact.
- Heating breaks down the albumin protein in egg whites, causing them to solidify into a semisolid mass. When making meringue, the strong physical action of an eggbeater achieves nearly the same result.
- By attaching themselves to functional groups on the surface of proteins, heavy metal poisons like lead and cadmium alter the structure of proteins.
- Denaturation of proteins can be done by bringing in physical changes as well as the introduction of chemicals. Most of the denaturation processes are irreversible, but it has been seen (in very few cases) that some of the denaturation processes can be reversed; it is then called renaturation of protein.
- The coagulation of egg white when an egg is boiled is one of the frequent instances of denaturation of proteins. Here, the temperature change causes denaturation.
- Another example of denaturation of proteins is curdling of milk, which happens when bacteria produce lactic acid.
Uses of denaturation of protein
Denaturation of proteins is generally considered disruptive to their natural function:
- Food Processing: Denaturation can increase the digestibility of some proteins during cooking. For example, when egg whites are cooked, their denaturation causes them to solidify, altering their texture and digestibility. Additionally, by eliminating dangerous bacteria, this process makes food safer to eat.
- Biotechnological Applications: Denaturation is used in various laboratory techniques, such as Western blotting, SDS-PAGE (Polyacrylamide Gel Electrophoresis), and other protein analysis methods. It’s often a necessary step in these processes to study and manipulate proteins.
- Pharmaceuticals: Some pharmaceuticals are made using denaturation. Denatured proteins from bacteria or viruses, for example, are used in vaccines to induce an immune response without causing the disease.
- Eliminating Biological Activity: Certain proteins’ activity can be intentionally decreased or removed by denaturation. This can be useful for specific medical procedures or situations where it’s necessary to inhibit the activity of proteins.
- Industrial Applications: Denaturation has applications in the textile industry. For example, to produce leather, certain proteins in animal skins must be denatured in order to produce materials that are durable.
Learn more
Crude protein content determination by the Kjeldahl method – The Science Notes