Definition
Western blotting is a widely used technique in molecular biology and immuno-genetics for the detection and analyses of proteins.
This method is also called “immune blotting” because of its nature to use an antibody for specifically identifying its antigen and also protein blotting.
A qualitative and semi quantitative data can be produced using western blotting for the desired protein of interest.
Principle of Western Blotting
- Western blotting technique principle relies on the specificity of binding between a molecule of interest and a probe to allow detection of the molecule of interest in the mixture of many other similar molecules.
- Here, molecule of interest is protein whereas the probe is typically an antibody raised against that particular protein.
- SDS PAGE is a prerequisite for western blotting .
- Proteins get spilt up by their size by a process called SDS-polyacrylamide gel electrophoresis.
- A primary antibody (an antibody precisely for the target protein) is then used to probe and wash the membrane with transferred protein.
- This primary antibody treated membranes are then reacted with a secondary antibody, usually an antibody enzyme conjugate (e.g. horseradish peroxidase).
- The target protein is visualized as band on blotting paper, X-ray film or imaging system.
Procedure of Western Blotting
Sample Preparation
- Use different samples to extract protein(e.g. tissues or cells)
- Use homogenizer or sonication to breakdown samples.
- Prevent sample digestion at cold temperature through protease and phosphates.
- Finally, observe concentration of proteins and use spectrophotometer for protein concentration.
Gel Electrophoresis
- Proteins are separated on the basis of their size, shape and charge.
- In SDS gel electrophoresis, protein samples are separated according to their molecular weights.
- Load protein samples to polyacrylamide gel (Higher percentage of gels are used for low molecular weight problems and vice versa).
Protein Transfer
- A solid support membrane is placed where we transfer the separated protein for antibody detection.
- The method is called electro blotting where an electric field is directed perpendicular to the surface of the gel maintain their relative position.
- A transfer sandwich is created made up of: a fiber pad (sponge), filter papers, blotting membrane, gel, filter papers and finally a fiber pad.
Protein staining
- The gels must be stained as proteins are not directly visible in the gel.
- Dyes like coomassie blue, silver stain or deep purples are used.
- The gel is imaged with suitable instrument and a permanent record can be made after staining.
Blocking
- Prevents non-specific binding of antibodies by blocking unoccupied sites of membrane with inert protein or non-ionic detergent.
- BSA and non-fat dry milk are commonly used typical blockers.
- Blocking agents should possess greater connection towards membrane than the antibodies.
Antibody probing
- Incubate the blot with one or more antibodies
- Primary antibodies are specific depending on the antigens to be detected.
- The secondary antibody (monoclonal or polyclonal) is linked to an enzyme that is used to indicate the location of the protein.
Washing
- Removes unbounded antibodies from the membrane.
- Commonly used buffer : a dilute solution of tween-20 in TBS or PBS buffer .
Protein Detection
- Alkaline phosphates (AP) and horse radish peroxidase (HRP) are widely used.
- Four types: Chromogenic detection, Chemiluminescence detection, Fluorescent detection and radioactive detection.
Analysis and Imaging
- Detection of signals using X-ray film, scanners or CCD.
- Benchmarking with marker protein to estimate the molecular weight of the protein.
- Verification can be done through qualitative and quantitative analysis to show the presence and absence of specific proteins of interests.
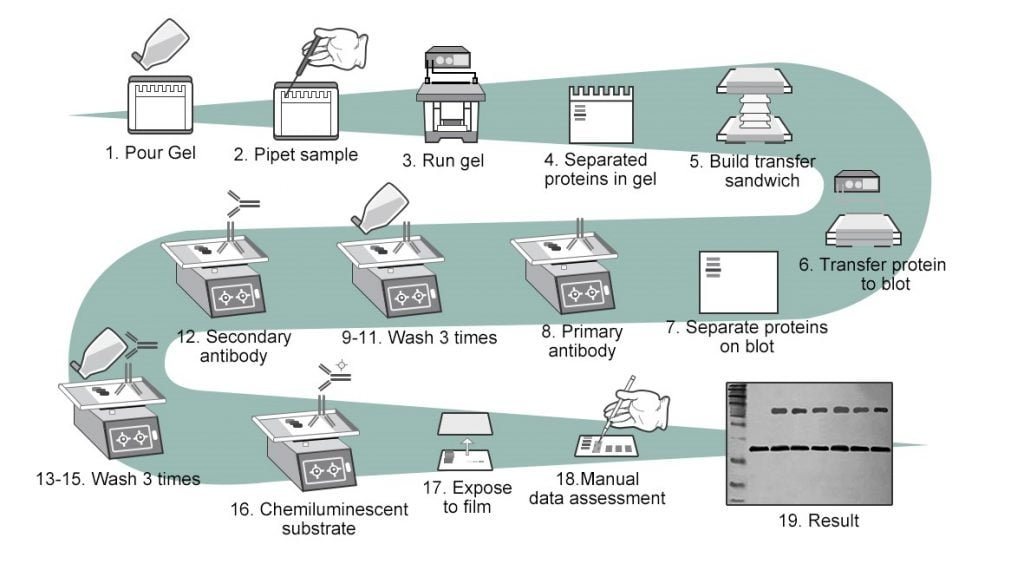
Figure adapted from: https://www.elabscience.com/List-detail-306.html
Applications
- Detection of particular protein from a mixture of proteins.
- Size and amount estimate of proteins in the mixture.
- Verification following a high sensitivity ELISA test for diagnosis of Lyme, HIV infection, BSE, HBV and so on.
- Detect condensed isoforms of proteins as well as tagged proteins.
Advantage and Disadvantages
Advantages
- Effective early diagnostic tool.
- Detect minimal immunogenic response form virus or bacteria.
- Requires fewer antibodies for testing.
- Detect specific protein from a large mixture of different proteins. (Even more than 300,000)
Disadvantages
- Requires specific primary antibodies to perform test on desired protein of interest.
- Challenging and hence requires well trained staffs.
- Poorer results as antibodies may revel off-target bindings.
- Detecting and imaging the results can be expensive as equipment cost is high.
References
- W.Neal Burnette (1981), “Western Blotting”: Electrophoretic Transfer of Proteins from Sodium Dodecyl Sulfate-Polyacrylamide Gels to Unmodified Nitrocellulose and Radiographic Detection with Antibody and Radio iodinated Protein A
- Yan Yang, Hongbao Ma (2009), Western Blotting and ELISA Techniques
- W. Neal Burnette (2009), Western Blotting : Remembrance of Things Past
- François Chevalier (2010), Standard Dyes for Total Protein Staining in Gel-Based Proteomic Analysis
- Tahrin Mahmood, Ping-Chang Yang (2015), Western Blot: Technique, Theory, and Trouble Shooting.
- Biji T. Kurien, R. Hal Scofield (2015), Western Blotting Methods and Protocols
- Advansta’s Step by Step Guide to Western Blots (Second edition)
- Brennan, John. “The Disadvantages of Western Blotting” sciencing.com, https://sciencing.com/disadvantages-western-blotting-8379318.html. 25 September 2020.