What is SDS-PAGE?
SDS – PAGE or sodium dodecyl sulfate-polyacrylamide gel electrophoresis is a technique most commonly used in genetic, biotechnology, biochemistry, and molecular biology laboratories for the separation of proteins from a mixed sample that identifies and quantifies a single protein from a mixture. They separate proteins on the basis of their electrophoretic mobility in which the mobility of the molecule is inversely proportional to its size and directly proportional to its charge.
SDS-PAGE is a widely used technique in biochemistry for protein separation and analysis.
During electrophoresis, proteins move towards an oppositely charged electrode while large-sized particle moves slowly due to its greater frictional and electrostatic forces exerted on it by the medium whereas small sized particle moves faster.
PAGE technique is often combined with a western blotting technique which uses antibodies to bind a specific protein antigen that helps in the identification of specific proteins with high specificity.
Principle of SDS-PAGE:
The main principle of SDS – PAGE is to separate specific proteins electrophoretically from a mixture of samples according to their size using a polyacrylamide gel matrix.
Polymerized acrylamide (polyacrylamide) is a gel-like matrix suitable for the separation of proteins which is a product of polymerization reaction between acrylamide and N, N’ –methylene-bis-acrylamide (BIS). The degree of cross-linking determines the hardness and softness of the gel which can be made by adjusting its concentration. The more the cross-linking, the harder will be the gel which slows down the migration of molecules but with the loose gel, the molecules migrate faster.
SDS is an anionic detergent which binds to protein backbone at a constant molar ratio and forms a negative charge which breaks the disulphide bonds of proteins disrupting the tertiary structure then the protein gets separated electrophoretically based on the length of their polypeptide chain.
Procedure and methodology of SDS-PAGE:
Sample preparation:
- The protein sample is prepared by heating them with SDS detergent and 2-mercaptoethanol until denaturation.
- SDS strongly binds to the protein and forms a negative charge while 2-mercaptoethanol frees sulfhydryl groups where polypeptide chains with an excess negative charge similar to mass ratio get released.
- With the protein sample, the buffer solution is added in microcentrifuge tubes and heat at 100°C for 3 minutes.
- Then the tubes are centrifuged at 15,000 rpm for 1 minute at 4°C.
- The supernatant is used further in SDS – PAGE processes.
Preparation of polyacrylamide gel:
- For preparing an electrophoretic gel, several components including acrylamide, N, N’ –methylene-bis-acrylamide (BIS), and a buffer solution is mixed together.
- During polymerization of the gel, ammonium persulfate, a free radical source, and a stabilizer is added and the mixture is degassed or butanol is added to prevent bubble formation.
- A comb is inserted between the spaces of the glass plate and allowed to polymerize.
- The polymerized gel is called a gel cassette.
Electrophoresis:
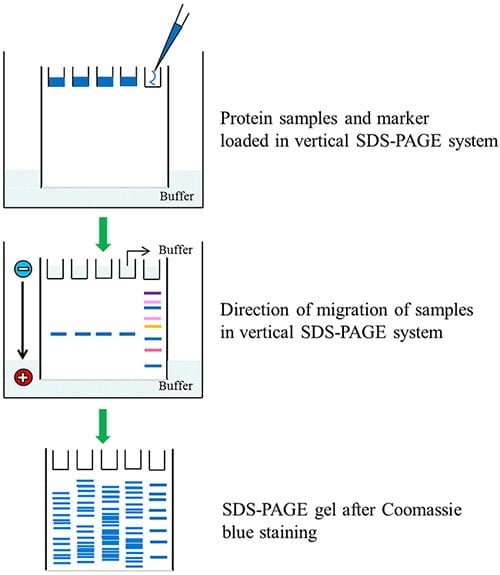
- The denatured 30 ml sample is pipette out and placed in the well and allowed to run at 30 mA for about one hour.
- As an electric current is applied, negatively charged protein molecules migrate towards a positively charged electrode through the gel.
- Each protein molecules also migrate at a different rate based on its molecular size, a small-sized molecule moves at a faster rate than that of the large-sized molecule.
- High voltage also increases the rate of migration.
- For protein molecules to be completely separated, it may take about one hour which is then stained and visualized.
Staining and visualization:
- Colored dyes such as Coomassie Brilliant Blue or ethidium bromide are used for staining the gel that gives a distinct colored band for proteins.
- The use of colored dyes is non-specific i.e, it targets all protein and these stains are non-reversible as well.
- This non-specific protein visualization is used for quick quantification of samples in a gel.
- Specific protein visualization requires the use of antibodies conjugated with a dye or enzyme that recognizes the unique structure of proteins.
- Western blotting is also associated with protein visualization by moving proteins from a gel to a membrane that can be probed with antibodies.
- The molecular mass of the unknown protein sample is determined by comparing the distance traveled by the unknown molecules with the marker.
(Source: https://www.sigmaaldrich.com/technical-documents/articles/biology/sds-page.html )
Advantages:
- Migration of the protein molecules is proportional to its molecular weight.
- SDS – PAGE is a highly sensitive test that separate molecules that have a minimum (~2%) difference in mass.
- Even small amount of sample is enough for processing.
- Pure DNA can be recovered from the gel.
- The pore size of the polyacrylamide gel can be controlled by adjusting the concentration of the two monomers.
Disadvantages:
- Gels are often difficult to prepare and takes a long time.
- Monomers used are potent neurotoxin chemical.
- Resolution of band is poor due to high alkaline operating pH.
- New gel is needed for each experiment.
Application of SDS – PAGE:
- SDS – PAGE is used in measurement of molecular weight of protein molecules.
- It is used in peptide mapping and in comparison of the peptide composition.
- It is used to estimate the protein purity and protein quantitation.
- It is used in post electrophoresis application; Western blotting.
- It is also used in the analysis of post-translational modification.
References:
- https://byjus.com/biology/sds-page/
- https://www.cleaverscientific.com/applications/polyacrylamide-gel-electrophoresis/
- https://www.news-medical.net/life-sciences/What-is-Polyacrylamide-Gel-Electrophoresis-(PAGE).aspx
- https://ruo.mbl.co.jp/bio/e/support/method/sds-page.html