Master staining techniques like Gram, Acid Fast Bacilli, Spore and Capsule Staining. Explore now about them!
“Stain” is a chemical reagent or dye that is responsible for the discoloration of the specimen. This use of stain for adding contrast for better vision of the microscopic image, giving a distinct view of the organism is called staining
– technique that is widely used for the examination of cells, tissues, and cellular components.
Smear preparation
- The purpose of making a smear is to fix the bacteria onto the slide and to prevent the sample from being lost during a staining procedure. A smear can be prepared from a solid or broth medium. Below are some guidelines for preparing a smear:
- Take a clean, grease-free slide and label it (on the left-slide usually)
- Place a small portion of an isolated colony (from solid medium) or a loopful of liquid bacterial culture at the center of the slide.
- In the case of solid culture, add a loopful of distilled water and using a sterile loop, spread the mixture covering about half of the total slide area.
- Air – dry the smear and after proper drying, the slide is ready for staining procedure.
Types of staining
Simple Staining
- Colouration of microorganisms by applying single dye to a fixed smear.
- It covers the fixed smear with stain for a specific period, after which this solution is washed off with water and slide blotted dry.
- Basic dyes like crystal violet, methylene blue, or carbol fuchsin are frequently used
- Done to determine the size, shape and arrangement of bacteria
Differential staining
- slightly more elaborate than simple staining techniques that the cells may be exposed to more than one dye or stain. E.g.
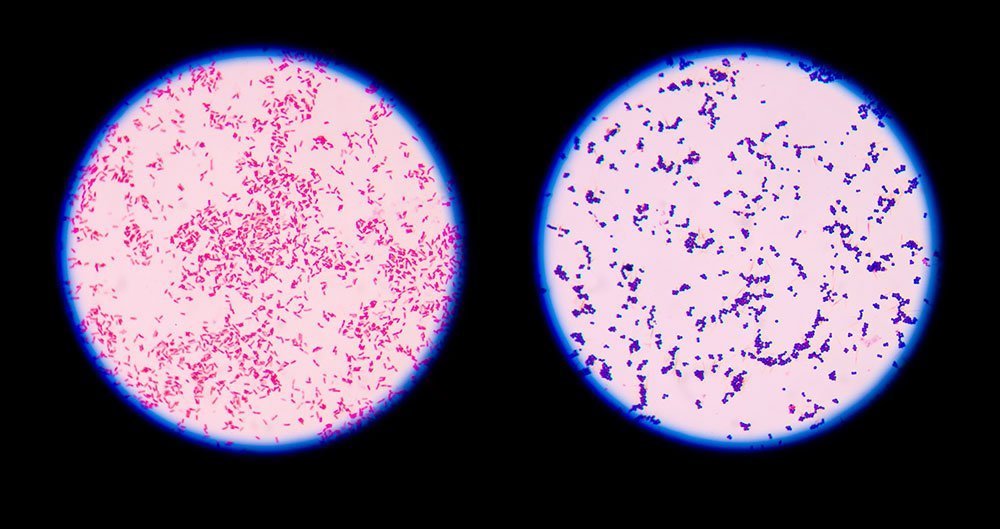
A. Gram Staining
- This technique was introduced in 1884 by Danish Physician Christian Gram
- Gram staining is a common technique used to differentiate two large groups of bacteria based on their different cell wall constituents.
- The Gram stain procedure distinguishes between Gram-positive and Gram-negative groups by coloring these cells red or violet.
Principle of Gram staining
The higher amount of lipid in the outer membrane in the G –ve cell wall is readily dissolved by alcohol, resulting in the formation of a large pore in the cell wall, thus facilitate leakage of crystal- violet – iodine (CV-I) complex, resulting in decolorization of the bacterial cell. Which later take counterstain (safranin) and appears red. In contrast, the cell wall of G +ve bacteria is thick and chemically simple. When treated with alcohol, it causes dehydration and closure of cell wall pore, thereby does not allow the loss of (CV-I) complex and cell remain purple.
Steps of Gram Staining
- The heat-fixed smear is flooded with basic dye crystal violet (Primary stain) and washed off slightly with tap water after 1min.
- Iodine solution is added to the smear and again after 1min, washed off indirectly for 2 sec with tap water.
- The smear is next decolourized by washing with ethanol (15secs). (This step generates the differential aspect of Gram stains. Gram positive bacteria retain crystal violet and Gram negative become colourless.)
- Finally smear is counter-stained for 1min with a simple basic dye different in color from Crystal violet. Safranin is the most common counter stain
- Wash off the slide with tap water after 1min and keep it on a draining rack till it dries
- Observe under microscope at 10x, 40x and then at 100x under oil immersion (with a drop of oil on the top of smear)
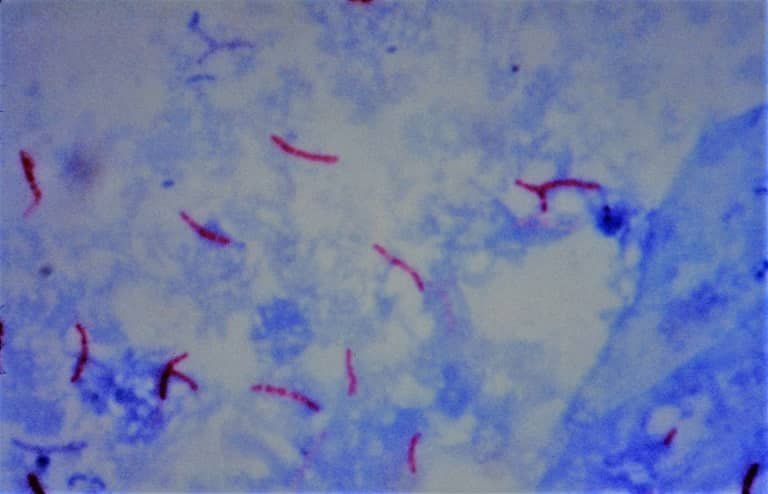
B. Acid Fast Bacilli Staining (AFB)
- first developed by Ziehl and later on modified by Neelsen, so also called Ziehl-Neelsen staining technique.
- main aim is to differentiate bacteria into acid fast (not decolorized by acid) group and non-acid fast groups; particularly the member of genus Mycobacterium (M. tuberculosis) are resistant and can only be visualized by acid-fast staining.
Principle of AFB staining
When the smear is stained with carbol fuchsin, it solubilizes the lipoidal material present in the Mycobacterial cell wall but by the application of heat, carbol fuchsin further penetrates through the lipoidal wall and enters into the cytoplasm. Then after all cell appears red. Then the smear is decolorized with a decolorizing agent (3% HCL in 95% alcohol) but the acid-fast cells are resistant due to the presence of large amounts of lipoidal material in their cell wall which prevents the penetration of the decolorizing solution. The non-acid fast organism lacks the lipoidal material in their cell wall due to which they are easily decolorized, leaving the cells colorless. Then the smear is stained with a counterstain, methylene blue. Only decolorized cells absorb the counterstain and take its color and appear blue while acid-fast cells retain the red color.
Steps of AFB staining
- Prepare bacterial smear (using a small bamboo stick) on a clean and grease-free slide, using a sterile technique.
- Allow smear to air dry and then heat fix.
Alcohol-fixation (5% phenol in ethanol): This is recommended when the smear has not been prepared from sodium hypochlorite (bleach) treated sputum and will not be stained immediately. M. tuberculosis is killed by bleach. Heat-fixation of untreated sputum will not kill M. tuberculosis whereas alcohol-fixation is bactericidal. - Cover the smear with carbol fuchsin stain.
- Heat the stain until vapor just begins to rise (i.e. about 60•C). Do not overheat. Allow the heated stain to remain on the slide for 5 minutes.
- Wash off the stain with clean water.
- Cover the smear with 3% v/v acid alcohol for 5 minutes or until the smear is sufficiently decolorized, i.e. pale pink.
Caution: Acid alcohol is flammable, therefore use it with care well away from an open flame. - Wash well with clean water.
- Cover the smear with malachite green stain for 1–2 minutes, using the longer time when the smear is thin.
- Wash off the stain with clean water.
- Wipe the back of the slide clean, and place it in a draining rack for the smear to air-dry (do not blot dry).
- Examine the smear microscopically, using the 100 X oil immersion objective.
C. Spore Staining
- All bacteria cannot form spores; takes place in some bacterial genera to withstand unfavorable conditions.
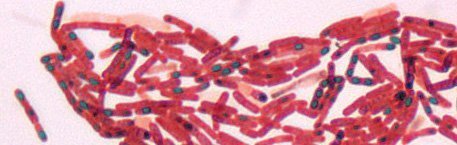
- In the Schaffer-Fulton procedure, endospores are first stained by heating bacterial smear with malachite green, which is a very strong stain that can penetrate endospores.
- After malachite green treatment, the rest of the cell is washed with water and is counter-stained with safranin. This technique yields a green endospore with a red vegetative cell.
D. Capsule staining
A capsule is a gelatinous outer layer secreted by bacterial cell and that surrounds and adheres to the cell wall
- A type of differential stain which uses acidic and basic dyes to stain background & bacterial cells respectively so that presence of capsule is easily visualized.
- In India Ink method a small drop of India Ink or Nigrosin is placed on the slide and using sterile technique, a thin bacterial smear is made; let stand for 5-7 minutes for air dry (no heat fix)
- Flood the smear with crystal violet stain (this will stain the cells but not the capsules) for about 1 minute. Drain the crystal violet by tilting the slide and let the stain run off until it air dries.
- Examine the smear microscopically (100X) for the presence of encapsulated cells as indicated by clear zones surrounding the cells.