The Ames Salmonella/microsome mutagenicity assay
History
The Ames Test is a popular bacterial assay used to determine if substances have the potential to cause mutations. At the University of California, Berkeley, Dr. Bruce Ames and his associates created the test in the early 1970s. The Ames test was created, and since then, it has grown into a crucial tool for identifying possible mutagens and carcinogens.
Dr. Ames, who received the National Medal of Science in 1991 for his contributions to the science of biochemistry, is honored by having the test take his name. Many significant tests and techniques utilized in contemporary health and environmental research have been developed as a result of Dr. Ames’ work.
Principle of Ames Test
Ames test is a bacterial assay employed to assess the mutagenesis potential of substances. The test is based on the principle that substances that are mutagenic can alter the DNA of bacteria leading to mutation, causing colonies to grow on media that is low in an important nutrient.
A strain of Salmonella typhimurium bacterium that is unable to synthesize the essential amino acid histidine is used in the test. The bacteria are grown on a specific medium lacking of histidine and with little nutrients. The next step involves exposing the bacteria to a test material, which is often a chemical or a combination of chemicals.
If the test material is mutagenic, it can cause mutation in the bacteria’s DNA, which may result in histidine synthesis. The bacteria can therefore develop colonies on the histidine-deficient media. The number of colonies that grow is a sign of how mutagenic the test substances is.
The test is often conducted using a variety of S. typhimurium strains, each of which has a unique mutation that increases its susceptibility to mutagens. These strains are more vulnerable to mutagens because of mutations in genes involved in the repair of DNA damage.
The Ames test is based on the principle that mutagens may harm genetic material, resulting in mutations in bacterial DNA. The test can evaluate whether a chemical has the ability to result in alterations that let the bacteria to thrive and build colonies by employing bacteria that are lacking in an important nutrient.
Ames Test Procedure
The Ames test is a bacterial assay used to assess a substance’s mutagenesis potential. The test makes use of a strain of Salmonella typhimurium bacterium that is incapable of producing the essential amino acid histidine. Here is a detailed procedure for conducting out the Ames test:
- Select the proper strain of the Salmonella typhimurium bacterium to be tested. The strains TA98 and TA100 are utilized the most frequently.
- Make a minimum agar medium without the histidine, biotin, and other ingredients necessary for bacterial growth. Through autoclaving, sterilize the media.
- Set up the test material in an appropriate solvent. Additionally, prepare both positive (Sodium Azide)and negative controls.
- A culture of Salmonella typhimurium bacteria should be inoculated in a nutrient-rich broth medium and incubated at 37°C until it enters the logarithmic phase of growth. S9 extract / liver extract is used to initiate the metabolism.
- Using a sterile spreader, distribute a tiny amount of the bacterial culture uniformly across the surface of the minimal agar medium.
- Apply a filter disc to the surface of the agar medium that has been impregnated with either the test substance or the control material. The plates should be incubated for around 48 hours at 37°C.
- Count the number of colonies that have developed on the minimum agar medium following the incubation time. A larger number of colonies than the negative control suggests mutagenicity, according to the results.
- Calculate the mutagenic index (MI) for each plate using the following formula:
MI = (Number of colonies on test plate)/ (Number of colonies on negative control plate)
9. The MI of the test substance should be compared to the MI of the positive control. The substance is regarded as mutagenic if the test substance’s MI is higher than the positive control’s.
The Ames test is a rapid, easy, and economical way to determine a substance’s mutagenic potential. However, it has significant drawbacks, such as the inability to identify all forms of mutagens and the lack of knowledge on the test substance’s metabolism. Despite these drawbacks, the Ames test is still a popular technique for determining if substances are mutagenic.

Ames Test Laboratory Protocol
Here is a laboratory protocol for performing the Ames test:
Materials:
- Bacterial strains (e.g., Salmonella typhimurium TA98 and TA100)
- Test chemical (dissolved in a suitable solvent)
- Agar plates (e.g., minimal glucose agar or Vogel-Bonner medium)
- Histidine-free media (e.g., minimal media)
- Sterile filter paper discs (6 mm diameter)
- Sterile saline solution (0.9%)
- Sterile pipettes and tips
- Incubator (37°C)
- Sterile forceps
Procedure:
- Transfer the bacterial strains (TA98 and TA100) from the frozen stock to nutrient agar plates, and then incubate them there for 24 hours at 37°C.
- To prepare the test material, dissolve it in an appropriate solvent (such as water or DMSO) in the concentration range that will be examined.
- To prepare the histidine-free media, dissolve the necessary quantity of minimum media in water, then autoclave the solution to sterilize it.
- To make the agar plates, put 20 mL of Vogel-Bonner medium or minimum glucose agar onto sterile Petri dishes and let it set up.
- Place a filter paper disc and 0.1 mL of the test chemical at the specified concentration onto each agar plate using sterile forceps.
- After the filter paper disc has dried, spread 0.1 mL of bacterial culture over it.
- For 48 hours, incubate the plates at 37 °C.
- Count the number of colonies on each plate after incubation, and then compare them to the results of the negative and positive controls.
- Determine how many revertant colonies are present on each plate and contrast them with the control plates.
- To ascertain the test chemical’s mutagenic potential, analyze the data and interpret the findings.
Note: Proper safety precautions should be taken when handling chemicals and bacteria, and all procedures should be performed in accordance with standard laboratory protocols.
Result Interpretation
The number of revertant colonies (colonies that have returned to the wild type phenotype) seen on the agar plates is often used to interpret the findings of the Ames test. The quantity of revertant colonies is employed as a measure of the test substance’s mutagenesis potential. The general processes for deciphering findings from an Ames test are as follows:
- Determine the typical number of revertant colonies on each test material concentration plate, and then compare these numbers to those for the negative and positive controls.
- The test substance is regarded as non-mutagenic if the number of revertant colonies in the test samples is comparable to the negative control (i.e., there is no appreciable rise in the number of colonies).
- The test material is regarded as mildly mutagenic if the number of revertant colonies in the test samples is noticeably greater than the negative control but comparable to the positive control.
- The test material is regarded as extremely mutagenic if the number of revertant colonies in the test samples is noticeably higher than the positive control.
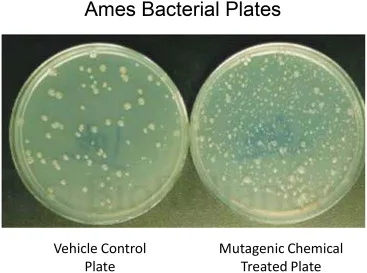
It is essential to remember that the Ames test is only a preliminary screening assay, and further research may be necessary to establish a substance’s ability to cause mutations. When determining the safety of a chemical, the Ames test results should also be evaluated in light of additional toxicological and environmental considerations.
Applications
The Ames test is a popular bacterial assay with a wide range of uses in industries including toxicology, pharmaceuticals, and environmental monitoring. Here are a few of the key uses for the Ames test:
- Assessment for mutagenic potential: The Ames test is frequently used to check the mutagenic potential of substances. The test can identify compounds that alter the DNA of bacteria, which may be a mutation that the substance is possibly mutagenic and carcinogenic.
- Drug discovery and development: The Ames test may be used to check mutagenicity in possible drug candidates. The chance of developing cancer can be raised by mutagenic drugs’ ability to destroy genetic material. Prior to testing on either animals or people, the test can aid in the early identification of possible mutagenic medicines.
- Environmental monitoring: The Ames test may be used to determine if environmental pollutants such air and water pollutants have the potential to cause mutations. The test can assist in locating compounds that might be risky for both human and environmental health.
- Food safety: The mutagenic potential of food additives, such as preservatives and colorings, may be assessed using the Ames test. Prior to being given the go-ahead for usage, the test can assist assure the safety of food additives.
- Regulation: To assess the safety of chemicals, regulatory organizations like the US Environmental Protection Agency (EPA) frequently employ the Ames test. The test’s findings can be used to assess the hazards that chemicals may pose and to establish standards for their usage.
Overall, the Ames test is a useful instrument for determining possible risks to human health and the environment as well as for assessing the mutagenic potential of substances. The test has a wide range of applications and has improved public health and safety.
Limitations of Ames Test
The Ames test has various drawbacks that should be taken into account, while being a popular and trustworthy assay for determining a chemical’s mutagenesis potential. The Ames test has the following primary drawbacks:
The Ames test has various drawbacks that should be taken into account, while being a popular and trustworthy assay for determining a chemical’s mutagenesis potential. The Ames test has the following primary drawbacks:
- Limited to identifying point mutations: It can only identify mutations that result in single nucleotide alterations or point mutations. Chromosome abnormalities or gene deletions cannot be detected, as can other kinds of mutations.
- Limited prediction: The Ames test does not always correctly indicate whether a substance would cause human cancer. Many substances that cause cancer in animals but not humans in the Ames test are mutagenic in humans.
- Limited data on metabolism: The test does not offer data on the substance’s metabolism, which may have an impact on its mutagenic potential.
- Limited tissue specificity: The test employs bacteria as a model organism, which could not accurately represent the mutagenic potential of a chemical in various organs or tissues of the body.
- Limited sensitivity to some forms of mutagens: The test may not be able to identify some mutagens that call for metabolic activation or that don’t result in mutations in the particular bacterial strains employed in the test.
- Interference from other compounds: A few substances might affect the test and result in falsely positive or falsely negative findings.
Despite these drawbacks, it is still a useful method for assessing the mutagenic potential of chemicals and locating possible risks to human health and the environment. To offer a more thorough evaluation of the safety of a chemical, the test should be used in combination with other tests and in vivo research.
What is the full form of Ames test?
The Ames Salmonella/microsome mutagenicity assay, often known as the “Salmonella test” or “Ames test,” is a short-term bacterial reverse mutation experiment created particularly to identify a variety of chemical agents that have the potential to cause genetic harm and subsequent gene changes.
Why is it called Ames Test?
The Ames test was designed by Dr. Bruce Ames in the early 1970s, and it is named for the scientist who developed it. The test was developed by Dr. Ames, a biochemist and molecular biologist at the University of California, Berkeley, to evaluate compounds for their ability to cause mutations quickly and affordably.
Why is liver extract used in Ames test?
Using a liver homogenate replicates the suspected mutagen’s metabolic breakdown in a mammalian system, improving predictions of the likelihood that compounds consumed by people would cause mutations.
Why sodium azide is used in Ames test?
Sodium azide is used as positive control. Sodium azide is a known mutagen which originates a back mutation, thus enabling the cells to grow and reproduce.