History of Mass Spectrometry (MS)
Mass spectrometry (MS) is an analytical method used to determine the mass-to-charge ratio of ions.The origins of mass spectrometry may be traced back to the early twentieth century, while physicists began researching the characteristics of ionized gases.
- J.J. Thomson discovered the very first mass spectrometer in 1913 while researching the characteristics of the cathode rays. His apparatus comprised of a cathode ray tube, a magnetic field, and a photographic plate. Thomson employed a magnetic field for separating ions depending on their mass-to-charge ratio, and a photographic plate to identify the separated ions.
- Francis Aston developed the first workable mass spectrometer in 1918, which he utilized to investigate the isotopes of various elements. Aston’s apparatus could separate ions with mass-to-charge ratios of up to 2,000, making it a substantial advance above Thomson’s.
- In the 1930s and 1940s, mass spectrometry was first applied in the study of organic molecules. A.B. Little and R. K. Hughes used mass spectrometry in 1938 to determine the molecular weight of numerous organic substances, including amino acids and carbohydrates.
- Mass spectrometry became widely utilized in the research of proteins and nucleic acids in the 1950s and 1960s. F.W. McLafferty and D.M. Stein used mass spectrometry to analyze the fragmentation patterns in peptides in 1955, which led to the emergence of the peptide mapping approach.
- Mass spectrometry proved more essential in biochemistry during the 1970s and 1980s. K. Biemann employed mass spectrometry for sequencing the first protein, insulin, in 1984.
Mass spectrometry is being used in many other fields, including proteomics, metabolomics, and forensic research. The approach has become an indispensable tool in the study of biological molecules, and it is still evolving as new technologies and applications emerge.
What is Mass Spectrometry?
Mass spectrometry (MS) is an analytical method for determining ion mass-to-charge ratios.
The basic principle of mass spectrometry is to ionize molecules, then separate and identify ions depending on their mass-to-charge ratio (m/z). Ionization, acceleration, separation, and detection are the four phases in the process.
Mass spectrometry may provide a variety of details regarding a molecule’s composition, structure, and attributes, such as its molecular weight, elemental content, and fragmentation patterns. It makes it a versatile tool for a variety of tasks, from detecting the components of a complicated mixture to identifying the molecular structure of a protein or nucleic acid.
Principle of Mass Spectrometry
The basic principle behind mass spectrometry (MS) is that atoms and molecules may be ionized and separated depending on their mass-to-charge ratio (m/z).
The process involves five steps: ionization, acceleration, separation, detection, and Data Analysis.
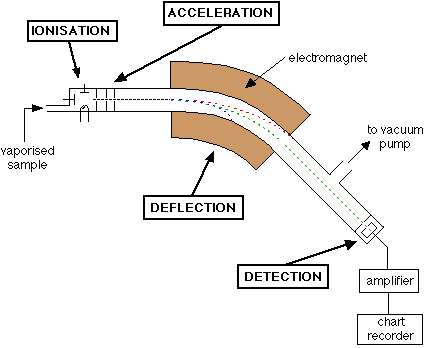
Ionization:
This is the first step in the mass spectrometry process. The sample is ionized, which breaks the molecules to electrically charged ions. The ionization technique utilized will be determined by the sample and the mass spectrometer. Electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), and matrix-assisted laser desorption/ionization (MALDI) are the most common ionization procedures.
Acceleration:
After ionizing the sample, the ions are propelled in an electric or magnetic field, providing ions kinetic energy proportionate with their charge & potential difference. The acceleration stage ensures that the ions travel at a constant velocity prior to entering the mass analyzer.
Separation:
A mass analyzer is used to distinguish the separated ions based on the ratio of mass to charge (m/z). The most popular types of mass analyzers consist of quadrupole, time-of-flight (TOF), ion trap, and Fourier transform ion cyclotron resonance (FT-ICR). Each mass analyzer utilizes a distinct technique to distinguish ions depending on their m/z ratio.
Detection:
Finally, using a detector such as a photomultiplier tube (PMT), electron multiplier (EM), or Faraday cup, the separated ions are identified and documented. The detector generates an electrical signal proportional to the number of ions observed, enabling the mass spectrum to be constructed, which displays the amount of each ion as an indication of its m/z ratio.
Data Analysis:
After generating the mass spectrum, the data has to be evaluated to identify the molecules contained in the sample. This involves correlating the mass spectrum to a database containing known spectra, deconvolving and identifying peaks in the spectrum using software, or manually analyzing the peaks in the spectrum in order to identify the compounds present.
The resultant mass spectrum offers substantial details about the identification, content, and structure of the molecules in the sample, making mass spectrometry a significant analytical tool in a variety of areas.
Components of Mass Spectrometry
The main components of a mass spectrometer (MS) include:
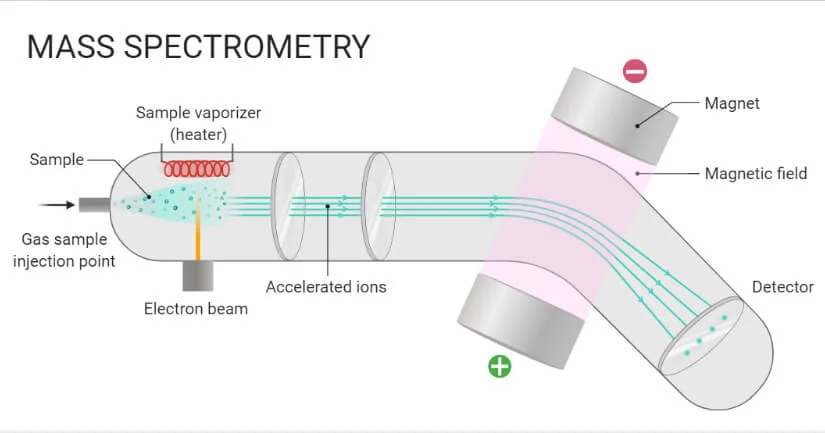
Ion Source:
The sample is ionized here, transforming its molecules into charged ions. The ion source utilized will be determined by the mass spectrometer and the material being examined. Electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), and matrix-assisted laser desorption/ionization (MALDI) are all common ionization generators.
Mass Analyzer:
The ions are sorted here depending on their mass-to-charge ratio (m/z). Mass analyzers are classified into various categories, each with its unique way of distinguishing ions. Quadrupole, time-of-flight (TOF), ion trap, and Fourier transform ion cyclotron resonance (FT-ICR) analyzers are examples.
Detector:
The ions are detected and recorded using a detector after they have been separated by the mass analyzer. The detector produces an electrical signal proportionate to the number of ions detected, enabling the mass spectrum to be constructed, which displays the quantity of each ion as a function of its m/z ratio. Photomultiplier tubes (PMT), electron multipliers (EM), and Faraday cups are examples of frequently used detectors.
Data System:
The data system is the one in control of collecting, processing, and storing the mass spectrometer’s output. It might contain software to operate the device, collecting and analyzing data, and producing reports.
Vacuum System:
A vacuum system is necessary for MS since it functions at low pressures because oxygen and other gases may interfere with the ionization & detection processes. Pumps, valves, and other parts may be included in the vacuum system to maintain the necessary vacuum level.
Sample Introduction System:
The sample is introduced via this mechanism into the mass spectrometer’s ion source. Depending on the sample type and the kind of mass spectrometer being used, it could also comprise syringes, gas chromatographs, liquid chromatographs, and other tools.
Applications of MS
In several disciplines, including chemistry, biochemistry, pharmacology, environmental research, forensics, and materials science, mass spectrometry (MS) is an effective analytical tool. MS is frequently used for the following purposes:
Compound identification: Organic and inorganic chemicals, proteins, and peptides, as well as other compounds, are often identified and characterized in different types of samples using the mass spectrometry (MS) technique. Chemistry, biochemistry, and pharmacology are among the disciplines that often employ it.
Structural elucidation: The molecular weight, fragmentation patterns, and ionization behavior of substances may all be exploited by MS to shed light on their structures. It is possible to ascertain the compound’s structure and composition using this data.
Quantitative analysis: MS is a tool that may be used to quantitatively analyze materials’ chemical composition. Environmental science, food science, and clinical research are just a few of the industries that frequently employ it.
Proteomics and metabolomics: For the identification and measurement of proteins and metabolites in intricate biological samples, MS is widely employed in the fields of proteomics and metabolomics.
Drug discovery and development: To detect and describe potential drugs, ascertain their pharmacokinetic & pharmacodynamic characteristics, and track drug metabolism, MS is utilized in drug discovery and development.
Forensic analysis: In forensic analysis, trace evidence including narcotics, explosives, and biological contaminants are identified and examined using MS.
Environmental analysis: In environmental science, MS is used to locate and measure contaminants in samples of soil, water, and air.
Materials analysis: In materials science, the composition and structure of materials including polymers, metals, and ceramics are examined using MS.
These are only a few of the numerous MS applications. This method is an essential instrument in contemporary analytical chemistry because of its versatility and sensitivity.
Advantages and Disadvantages of MS
Advantages of Mass Spectrometry (MS):
- High sensitivity: MS is a useful instrument for many applications since it is very sensitive and can find extremely small amounts of chemicals in complicated samples.
- High selectivity: MS offers great selectivity and specificity in chemical identification since it is capable of distinguishing among molecules with similar masses.
- Structural information: Information on the structure and fragmentation of compounds may be obtained through MS, enabling their identification and structural elucidation.
- Quantitative analysis: It can be utilized to provide exact and precise assessments of sample concentrations.
- Wide range of applications: Several disciplines, including chemistry, biochemistry, pharmacology, and environmental science, make extensive use of MS.
Disadvantages of Mass Spectrometry (MS):
- Cost: The price of mass spectrometers can vary, as can the cost of maintenance and operation.
- Complexity: Mass spectrometry data processing and analysis can be challenging, demanding specialist knowledge and skills.
- Sample preparation: MS frequently calls for time- and labor-intensive sophisticated preparation of samples, methods as extraction, derivatization, and separation.
- Limited sample compatibility: Some samples might not be able to be analyzed using MS, necessitating extra processes or different analytical methods.
- Potential for interference: The precision and dependability of MS data can be impacted by matrix effects or co-eluting substances.
Learn more: