In the world of modern analytical chemistry, Nuclear Magnetic Resonance (NMR) spectroscopy stands as a beacon of innovation, providing scientists with invaluable insights into the molecular structure and dynamics of diverse compounds. This non-destructive technique has revolutionized the field, enabling researchers to delve deep into the mysteries of matter. In this article, we will explore the fascinating realm of NMR spectroscopy, its principles, working mechanism, instrumentation, applications, and the pivotal role it plays across various scientific domains.
Understanding NMR Spectroscopy: Principles and Fundamentals
NMR spectroscopy operates on the principles of nuclear magnetic resonance, where atomic nuclei resonate in a magnetic field when exposed to radiofrequency radiation. This resonance phenomenon is dependent on the chemical environment and magnetic properties of the nucleus. As a result, NMR spectroscopy has the unique ability to unravel the structural intricacies of organic, inorganic, and biological molecules.
Principle of NMR Spectroscopy: Spin and Resonance
At the core of NMR spectroscopy lies the intrinsic property of atomic nuclei called “spin.” Just as spinning tops wobble at specific frequencies based on their physical properties, atomic nuclei oscillate at characteristic frequencies within a magnetic field. When a sample is subjected to a strong external magnetic field and irradiated with radiofrequency pulses, the nuclei absorb energy and transition to higher energy states. As they relax back to their original states, they emit energy, which is detected as an NMR signal. These frequencies are recorded in an NMR spectrum, producing a detailed fingerprint of the molecule under study. The spectrum provides valuable information about the arrangement of atoms, connectivity, and even spatial orientations.
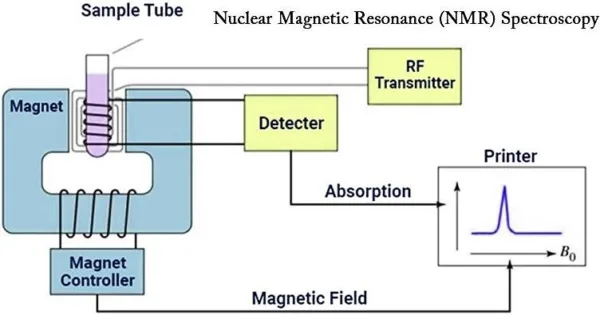
Instrumentation of NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy, a powerful analytical technique, relies on sophisticated instrumentation to reveal the intricate details of molecular structures and interactions. The evolution of NMR instrumentation has been a driving force in enhancing the sensitivity, resolution, and versatility of the technique. Here’s a comprehensive look at the key components and advancements that constitute the instrumentation of NMR spectroscopy:
1. Magnet System:
Central to NMR spectroscopy is the magnet system. High-strength superconducting magnets generate the static magnetic field required for nuclei to resonate. These magnets provide exceptional stability, crucial for achieving high-resolution spectra.
2. Radiofrequency (RF) System:
The RF system produces and controls the radiofrequency pulses used to excite and manipulate the nuclei. It consists of a transmitter for pulse generation and a receiver for detecting the resulting NMR signals.
3. NMR Probe:
The NMR probe holds the sample and is designed for optimal signal detection. Probes can be tailored for specific nuclei, sample types, and experimental conditions, maximizing sensitivity and signal-to-noise ratio.
4. Gradient Coils:
Gradient coils are used to spatially encode information in multidimensional NMR experiments. By applying magnetic field gradients across the sample, spatial information is obtained, enabling the determination of molecular connectivity.
5. Console and Computer:
The NMR console serves as the control center, where parameters like magnetic field strength, pulse sequences, and data acquisition settings are programmed. Data is collected and processed using software on a connected computer.
6. Pulse Sequences:
Pulse sequences determine the timing and sequence of RF pulses applied to the sample. These sequences create specific experimental conditions that yield information about molecular dynamics, interactions, and structures.
7. Cryogenic Probes:
Cryogenic probes are cooled to low temperatures using liquid nitrogen or helium, enhancing sensitivity by reducing thermal noise. This advancement has significantly improved signal quality and allowed for the study of challenging samples.
8. Multinuclear Capabilities:
Modern NMR instruments offer multinuclear capabilities, enabling the study of a variety of nuclei beyond hydrogen and carbon, including phosphorus, nitrogen, and fluorine.
9. Automation and Software:
Automation features simplify data collection and instrument operation. Advanced software streamlines data analysis, spectral processing, and the visualization of NMR spectra.
10. Hyphenated Techniques:
NMR can be coupled with other techniques such as mass spectrometry or chromatography, offering complementary information and expanding the analytical capabilities for complex samples.
11. Portable NMR Devices:
Recent developments include portable NMR devices that can be used in field applications, facilitating real-time analysis of samples without the need for specialized laboratory setups.
The instrumentation of NMR spectroscopy continues to evolve, driven by innovations in magnet technology, RF hardware, and data processing methods. As advancements continue, NMR spectroscopy remains at the forefront of scientific research, enabling insights into the fundamental properties of matter and pushing the boundaries of our understanding across various scientific disciplines.
Working Mechanism of NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy operates through a systematic series of steps that lead to the generation of valuable information about molecular structures and interactions. This intricate process can be outlined in the following comprehensive sequence:
1. Sample Preparation:
The NMR spectroscopy journey begins with meticulous sample preparation. The substance under investigation, often dissolved in a suitable solvent, is carefully placed within a precisely calibrated NMR tube or glass tube. The sample’s purity is of paramount importance, as any impurities could potentially interfere with the accurate interpretation of NMR signals.
2. Alignment in Magnetic Field:
The NMR tube containing the prepared sample is introduced into a strong external magnetic field. This magnetic field is fundamental to the entire NMR process. Within this field, the atomic nuclei of the sample’s constituent elements align themselves in relation to the direction of the magnetic field lines.
3. Irradiation with Radiofrequency Pulses:
The NMR magic commences with the application of radiofrequency (RF) pulses. These pulses are skillfully directed at the sample, with a precision that matches the resonant frequency of the specific nuclei of interest within the sample. The nuclei, under the influence of these RF pulses, absorb energy and transition from their lower-energy states to higher-energy states.
4. Relaxation and Signal Emission:
After absorbing energy and transitioning to higher-energy states, the excited nuclei eventually return to their original equilibrium states. This relaxation process is accompanied by the emission of energy in the form of radiofrequency signals. The emitted signals are exquisitely sensitive to the chemical environment of the nuclei, containing valuable information about their immediate surroundings.
5. Data Acquisition and Analysis:
The emitted radiofrequency signals, resonating with the unique chemical context of the nuclei, are captured and transformed into what is known as an NMR spectrum. This spectrum portrays a visual representation of the frequencies at which different nuclei resonate. Each peak within the spectrum corresponds to a specific type of nucleus within the sample. Through a meticulous process of analysis and interpretation, scientists can deduce an array of essential insights about the molecular structure, connectivity, and dynamic behavior of the sample.
The working mechanism of NMR spectroscopy is a carefully orchestrated dance between external magnetic fields, radiofrequency pulses, and the intrinsic properties of atomic nuclei. Through this elegant interplay, NMR spectroscopy unveils a world of molecular information, enabling scientists to decode the mysteries of matter in its various forms and applications.
The Role of NMR Spectroscopy in Chemical Analysis
NMR spectroscopy has found its niche in chemical analysis, becoming an indispensable tool for identifying and quantifying compounds in mixtures. Unlike other techniques, NMR does not require extensive sample preparation, making it a rapid and efficient method. Its high selectivity and sensitivity enable researchers to distinguish between compounds with similar structures, aiding in the detection of trace elements.
When compared to techniques like mass spectrometry, NMR has the advantage of providing insights into molecular dynamics. This is especially valuable in drug discovery, where understanding the interactions between small molecules and biomolecules is crucial. NMR spectroscopy allows scientists to monitor binding events, conformational changes, and enzymatic reactions in real-time.
Applications of NMR Spectroscopy
NMR spectroscopy finds applications across diverse fields:
- Chemistry: Determines molecular structures and functional groups.
- Biology: Studies protein and nucleic acid structures, interactions, and dynamics.
- Metabolomics: Identifies and quantifies metabolites in biological samples.
- Material Science: Characterizes composition and properties of materials.
- Quality Control: Verifies product authenticity and composition.
- Environmental Analysis: Detects pollutants and contaminants in air, water, and soil.
- Medical Diagnostics: Provides detailed images for disease diagnosis.
- Pharmacology: Studies drug-target interactions and mechanisms.
- Biological Interactions: Analyzes protein-ligand and protein-protein binding.
- Quantitative Analysis: Determines compound concentrations.
- Process Monitoring: Ensures consistency in production processes.
- Food Science: Analyzes food composition, flavor, and quality.
- Geochemistry and Archaeology: Studies minerals and artifacts.
Conclusion
Nuclear Magnetic Resonance (NMR) spectroscopy stands as a testament to human ingenuity in unraveling the secrets of matter. From elucidating the structure of organic molecules to probing the dynamics of biomolecules, NMR has reshaped the landscape of analytical chemistry. Its applications are vast and varied, impacting fields as diverse as chemistry, biology, material science, and environmental analysis. As technology continues to advance, NMR spectroscopy is poised to maintain its position as a cornerstone of scientific exploration, driving innovation and discovery forward.
References:
- Brown University – NMR Introductory Lecture
- Hebrew University of Jerusalem – What is NMR?
- Michigan State University – NMR Spectroscopy
- LibreTexts – NMR Spectroscopy
- Bruker – NMR 101
- Sigma-Aldrich – NMR Spectroscopy
- BYJU’S – NMR Spectroscopy
- Technology Networks – NMR Spectroscopy Principles
- University of Texas Medical Branch – NMR Spectroscopy