Surface plasmon resonance (SPR) is a label-free optical method used to examine molecular interactions in real-time. It relies on the phenomena of surface plasmons, which are electron oscillations that take place at the interface of a metal and a dielectric liquid, such air or water.
In SPR, a glass prism or the surface of a sensor chip are coated with a tiny coating of metal, usually gold or silver. The reflected light is then measured after a laser beam is pointed at a certain angle onto the metal-coated surface. The refractive index at the metal-dielectric interface changes as molecules bond to the metal surface, shifting the angle at which light is reflected as a result.

Without labeling or immobilizing the molecules of interest, it is feasible to ascertain the kinetics and affinities of biomolecular interactions, such as protein-ligand interactions or antigen-antibody binding, by observing these changes in the reflected light. This makes SPR an effective tool for studying biomolecular interactions, protein engineering, and drug development.
Principle of Surface Plasmon Resonance
In SPR, a glass prism or the surface of a sensor chip are coated with a thin metal layer, usually made of gold or silver. The reflected light is then measured after a laser beam is pointed at a certain angle onto the metal-coated surface. The refractive index at the metal-dielectric interface changes as molecules bond to the metal surface, shifting the angle at which light is reflected as a result. Real-time monitoring of this change reveals details about the affinities and dynamics of the interacting molecules.
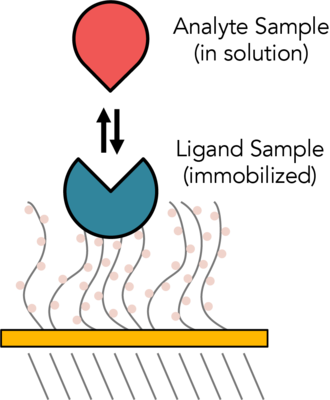
The refractive index of the medium on the sensor chip directly affects the SPR signal. The refractive index of the sensor surface varies as a result of biomolecule interaction. In an SPR experiment, binding to a second molecule (the Analyte) is monitored while the Ligand is immobilized on a sensor chip. Resonance units (RU) are used to measure responsiveness, and they are proportional to the mass on the surface and the number of molecules attached to the surface for any specific interactant. Real-time sensograms are shown once the response has been recorded. The kinetic binding constants (ka, kd) and equilibrium binding constants (affinity, Ka = 1/Kd) can both be measured using SPR studies.
Instrumentation of Surface Plasmon Resonance (SPR)
Surface Plasmon Resonance (SPR) apparatus entails a complicated set-up made up of a light source, optical components, and a detector. The main parts of an SPR system are as follows:

1. Light Source:
An SPR system requires a stable and high-intensity light source that can provide monochromatic light. The most commonly used light source is a low-power laser, typically with a wavelength of 632.8 nm or 785 nm, which is directed towards the surface of a prism or sensor chip.
2. Optical Elements
Mirrors, polarizers, and prisms, among other optical components, are employed in an SPR system to focus and polarize the light beam. In most cases, a prism is employed to couple light into the metal layer and produce plasmon waves.
3. Sensor Chip
An SPR system’s sensor chip is a crucial part. It includes a thin coating of ligand- or probe-coated metal, often made of gold or silver. An alteration in the angle of the reflected light, brought on by the binding of analyte molecules to the surface, is picked up by the detector.
4. Sample Handling System
The analyte solution is applied to the sensor chip using the sample handling system. The flow rate and volume of the sample solution are controlled using a flow cell or microfluidic system.
5. Detector
The detector is utilized to track modifications in reflected light brought on by analyte molecule attaching to the sensor surface. Usually, a photodiode or a charge-coupled device (CCD) camera is used.
6. Computer and Software
A computer uses specialized software to evaluate and process the detector’s output signal in real time, allowing for real-time monitoring and data processing.
In order to produce precise and trustworthy findings, an SPR system’s instrumentation must be carefully aligned and calibrated. But if the system is configured properly, it can offer useful data on the kinetics and affinity of biomolecular interactions.
Applications of Surface Plasmon Resonance
SPR has become a widely used tool in drug discovery, protein engineering, and biomolecular interaction studies. Here are some examples of its applications:
1. Drug Discovery
SPR analyzes the binding kinetics and affinity of drug candidates to target proteins in order to find and improve therapeutic candidates. It can also be used to check a vast library of chemicals for a target protein-binding capability. In the process of creating a cancer treatment, SPR was employed to examine the binding kinetics and affinity of a small molecule inhibitor to the target protein kinase.
2. Antibody Characterization
SPR is used to assess the affinity and kinetics of antibodies’ binding to their target antigens. For the creation of therapeutic antibodies and diagnostic tests, this information is essential. SPR was employed to investigate the kinetics and affinity of an antibody’s binding to its target antigen in the process of creating a therapeutic antibody for autoimmune disorders.
3. Protein-Protein Interactions
Protein interactions, which are crucial for many biological processes, are studied using SPR. It can be applied to investigate the mechanics of known interactions as well as discover new protein-protein interactions. SPR was used to investigate how proteins interact with their binding partners, which control how genes are expressed.
4. Enzyme Kinetics
The kinetics of enzyme-catalyzed reactions are investigated using SPR. The rate of product production can be used to calculate the kinetics and activity of an enzyme. The kinetics of an enzyme-catalyzed process that is crucial in the liver’s drug metabolism was investigated using SPR.
In conclusion, Surface Plasmon Resonance (SPR) is a potent and adaptable analytical method that has completely changed the world of molecular biology and biochemistry. SPR is a promising method for analyzing protein-protein, protein-DNA, and protein-ligand interactions since it can detect biomolecular interactions in real-time and without labeling. Additionally, SPR has a wide range of uses in environmental monitoring, medical diagnostics, and drug development. It is anticipated that SPR will become more significant in both basic and practical research as technology develops.
Lear more