Hemoglobin and Myoglobin were the first proteins to be successfully subjected to completely successful X-rays analysis by J. C. Kendrew and Max Perutz (Nobel Prize for Chemistry 1962). Haemoglobin is also known as tetrameric form of myoglobin, structurally and functionally more complex than myoglobin.
Hemoglobin are the members of conjugated protein (oxygen-globular binding proteins), a tetrameric allosteric protein containing globin with prosthetic group heme; found in erythrocytes which transports oxygen from the lungs to tissues.
Structure of Hemoglobin
Each subunit contains a
I. non-protein part
called a prosthetic group, which is called a heme, produced by the combination of iron with a porphyrin ring and
II. Globin
the apoprotein part.
Structure of Heme (prosthetic group)
- Heme is a derivative of porphyrin.
- Porphyrin: Porphyrins are cyclic compounds formed by the fusion of 4 pyrrole rings linked by methenyl bridges (=CH-). Since an atom of iron is present heme is a ferroprotoporphyrin. These rings are names as I,II,III, IV and the bridges are names as Alpha, beta, gamma and delta. Porphyrins contain side chains attached to each of the other four pyrrole rings. Different Porphyrins vary in the nature of the side chains that are attached to each of the pyrrole rings.
- Heme consists of one ferrous atom (Fe++) that is coordinated in the center of the tetra pyrrole ring of protoporphyrin IX. This iron atom has six coordination bonds, four to nitrogen atoms that are part of the flat porphyrin ring system and two are perpendiculars to the porphyrin. The coordinated nitrogen atoms (which have an electron-donating character) help prevent conversion of the heme iron to the ferric (Fe3+) state. One of these two coordination bonds is occupied by a side-chain nitrogen of a His residue. The other is the binding site for molecular oxygen (O2). Therefore, the oxidation of the heme iron is prevented by the presence of the distal histidine side chain preventing easy release of oxygen. This is an important mechanism to understand the regulation of hemoglobin’s affinity for oxygen as well.
- Iron in the Fe2+ state binds oxygen reversibly; in the Fe3 state it does not bind oxygen. Heme is found in a number of oxygen-transporting proteins, as well as in some proteins, such as the cytochromes, that participate in oxidation-reduction (electron-transfer) reactions. Molecules, like carbon monoxide (CO) and nitric oxide (NO), coordinate to heme iron with greater affinity than O2. When a molecule of CO is bound to heme, O2 is excluded, which explains toxicity of O2.
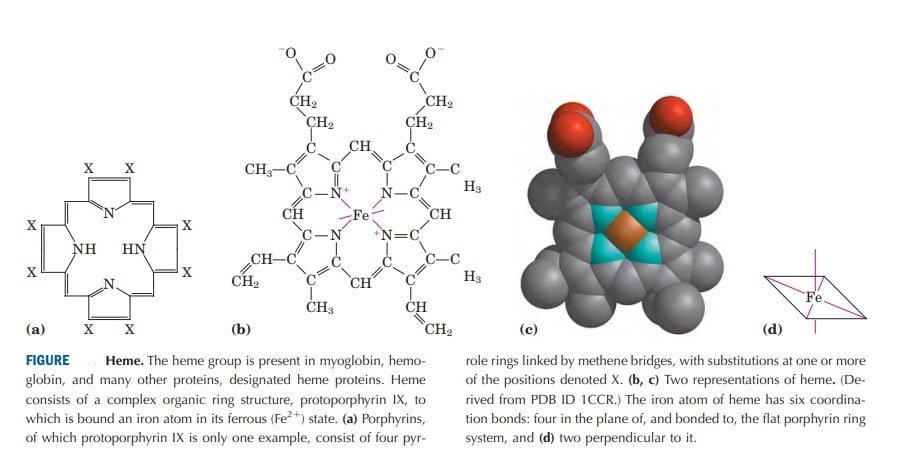
[Source: Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles of Biochemistry. New York : W.H. Freeman,]
Globin and the quaternary structure of hemoglobin:
- The hemoglobin comprises of the globin polypeptide chains of two dimers. Thus, heterotetrameric tetramer is composed of two identical dimers,(αβ)1 and (αβ)2.The two polypeptide chains within each dimer are held tightly together, primarily by hydrophobic interactions as well as the Ionic and hydrogen bonds. Though they are held together by polar bonds, the two dimers are able to move with respect to each other.
Conformation of hemoglobin
- T form: The deoxy form of hemoglobin is called the “T,” or taut(tense) form. In the T form, there are two αβ dimers interact through a network of ionic bonds and hydrogen bonds.These bonds restrict the movement of the polypeptide chains. The T form is the low oxygen-affinity form of hemoglobin.
- R form: The binding of oxygen to hemoglobin causes the rupture of some of the ionic bonds and hydrogen bonds between the αβ dimers. This leads to a structure called the “R,” or relaxed form, in which the polypeptide chains have more freedom of movement . The R form is the high oxygen-affinity form of hemoglobin

Forms of Hemoglobin
Besides adult hemoglobin (HBA1) as described above other minor hemoglobin’s such as HbA2 which are found less than 5%, Fetal Hemoglobin (HbF) are found during fetal development which are less then 2% and Glycosylated hemoglobin (HbA1c) occupies less than 5% in a normal human body.
| Forms of Haemoglobin | Hb A | Hb A2 | Hb F | HbA1C |
| Structure | α2β2 | α2δ2 | α2γ2 | α2β2-glucose |
| Normal % | 96-98 % | <5% | <2 % | <5 % |
Cooperative Binding of Oxygen
- The oxygen dissociation curve for hemo -globin is sigmoidal in shape indicating that the subunits cooperate in binding oxygen.
- Cooperative binding of oxygen by the four subunits of hemoglobin means that the binding of an oxygen molecule at one heme group increases the oxygen affinity of the remaining heme groups in the same hemoglobin molecule. This effect is referred to as heme-heme interaction.
- The net effect is that the affinity of hemoglobin for the last oxygen bound is approximately 300 times greater than its affinity for the first oxygen bound. The reason behind this is when O2 binds to the Fe2+ of hemoglobin, the shape of hemoglobin changes, which allows the hemoglobin to hold on to the oxygen until it is delivered to tissues. This change in shape is known as a conformational change.
- After the oxygen is delivered to the tissues, the shape of the hemoglobin changes back to its pre-oxygenated form. As a result of the cooperative binding, oxygen allows hemoglobin to deliver more oxygen to the tissues in response to relatively small changes in the partial pressure of oxygen.
Oxygen Transportation
Nearly all the oxygen carried by whole blood in animals is bound and transported by hemoglobin in erythrocytes (red blood cells). Normal human erythrocytes are small (6 to 9 m in diameter), biconcave disks. They are formed from precursor stem cells called hemocytoblasts. The main function of RBC is to carry hemoglobin at a very high concentration (~34% by weight) which is dissolved in the cytosol. In arterial blood, from the lungs through the heart to the peripheral tissues, hemoglobin is about 96% saturated with oxygen whereas in the venous blood returning to the heart, hemoglobin is only about 64% saturated.
Learn more about Components and Functions of Blood – The Science Notes
References
- Mozzarelli A., Bruno S., Ronda L. (2013) Biochemistry of Hemoglobin. In: Kim H., Greenburg A. (eds) Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40717-8_3
- Farid Y, Bowman NS, Lecat P. Biochemistry, Hemoglobin Synthesis. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536912/
- Harvey, Richard A., Ph. D. (2011). Lippincott’s illustrated reviews: Biochemistry. Philadelphia :Wolters Kluwer Health,
- Turdy- McKee, T., & McKee, J. R. (2012). Biochemistry: The molecular basis of life. Oxford: Oxford University Press.pp.171-175
- Lehninger, Albert L., Cox, Michael M. Nelson, David L. Lehninger Principles Of Biochemistry. New York : W.H. Freeman, 2008 pp158-161
- Alberts, Bruce, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Molecular Biology of the Cell. New York: Garland Science, 2002.pp 147-148, 760
- Lodish, Harvey F. Molecular Cell Biology. New York: W.H. Freeman and Co, 5th edition.pp-82-83
- https://www.barnardhealth.us/glucose-phosphate/oxygen-can-be-bound-to-a-heme-prosthetic-group.html
- https://accesspharmacy.mhmedical.com/content.aspx?bookid=1696§ionid=111398218
- https://www.cliffsnotes.com/study-guides/biology/biochemistry-i/oxygen-binding-by-myoglobin-and-hemoglobin/hemoglobin-and-myoglobin
- https://www.ucsfhealth.org/education/hemoglobin-and-functions-of-iron
- https://www.drawittoknowit.com/course/biochemistry/glossary/biochemical-pathway/hemoglobin-myoglobin-3-hemoglobin
- https://www.medicalnewstoday.com/articles/318050